Case Report
2022
March
Volume : 10
Issue : 1
Primary Ewing sarcoma of the mesentery: An extremely uncommon case
Patra S, Trivedi P, Nagarjun BR
Pdf Page Numbers :- 44-46
Sanjiban Patra1, Priti Trivedi1,*, and Nagarjun BR1
1Department of Oncopathology, The Gujarat Cancer Research Institute, Ahmedabad, Gujarat 380016, India
*Corresponding author: Dr. Priti Trivedi, Department of Oncopathology, The Gujarat Cancer Research Institute, New Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat 380016, India. Email: priti.trivedi@gcriindia.org
Received 8 September 2021; Revised 11 November 2021; Accepted 19 November 2021; Published 26 November 2021
Citation: Patra S, Trivedi P, Nagarjun BR. Primary Ewing sarcoma of the mesentery: An extremely uncommon case. J Med Sci Res. 2022; 10(1):44-46. DOI: http://dx.doi.org/10.17727/JMSR.2022/10-10
Copyright: © 2022 Patra S et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Ewing sarcoma usually arises in the bones of children. Extra-skeletal Ewing sarcoma commonly occurs in soft tissue and rarely involves other organs of the body. The mesentery besides the intestine is an extremely uncommon location. The diagnosis poses a challenge as other malignant small round cell tumors come in the differential diagnosis in this location. Early histopathological diagnosis and immunohistochemical confirmation are crucial to outline an appropriate treatment plan. Expression of membranous CD99 and nuclear FLI-1 and NKX2.2 are fairly specific for diagnosis. The molecular study is reserved for difficult cases. Prognosis is poor in an extra-skeletal location in older individuals.
Keywords: Ewing sarcoma; extra-skeletal; mesentery; CD99; NKX2.2
Full Text
Introduction
Ewing sarcoma (ES) most commonly occurs in bones in children but also common in soft tissues in young adult. Approximately 90% of ES carry the genetic EWS-friend leukemia virus integration-1 (FLI1) fusion protein resulting from a specific t (11;22) translocation [1]. It is extremely uncommon in mesentery [2, 3]. The diagnosis of skeletal as well as extra-skeletal ES is confirmed usually by immunohistochemistry (IHC) and molecular study in difficult cases. Here we describe a case of a primary mesenteric ES in an adult patient.
Case description
A 40-years-old gentleman presented with abdominal pain and fullness. Clinically a lump was palpable in left hypochondrium extending to left iliac fossa. He had no past significant medical or surgical history. Routine investigations, serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA19-9) were within normal limits. Contrast enhanced computed tomography (CECT) scan of abdominopelvic region showed the presence of approximately 16×13×12 cm well-defined heterogeneously enhancing soft tissue mass with internal necrosis, arising from the mesentery in left lumber region and abutting adjacent bowel loops, left psoas muscle, abdominal aorta, left renal vessels and left lateral abdominal wall (Figure 1a). Other abdominal organs including kidney and adrenal were normal. Thoracic organs were also normal. A CT guided core needle biopsy was done. Histopathology revealed a malignant small round cell tumor, morphologically non-lymphoid. Tissue was inadequate for immunohistochemical confirmation. Hence, an exploratory laparotomy and excision of the mass was performed for histopathological study and definite typing of the tumor. Grossly, the soft tissue mass was well-circumscribed with attached glistening peritoneum on surface (Figure 1b). Cut surface was variegated, soft to firm to spongy appearance along with scattered blood-filled cystic spaces (Figure 1c).
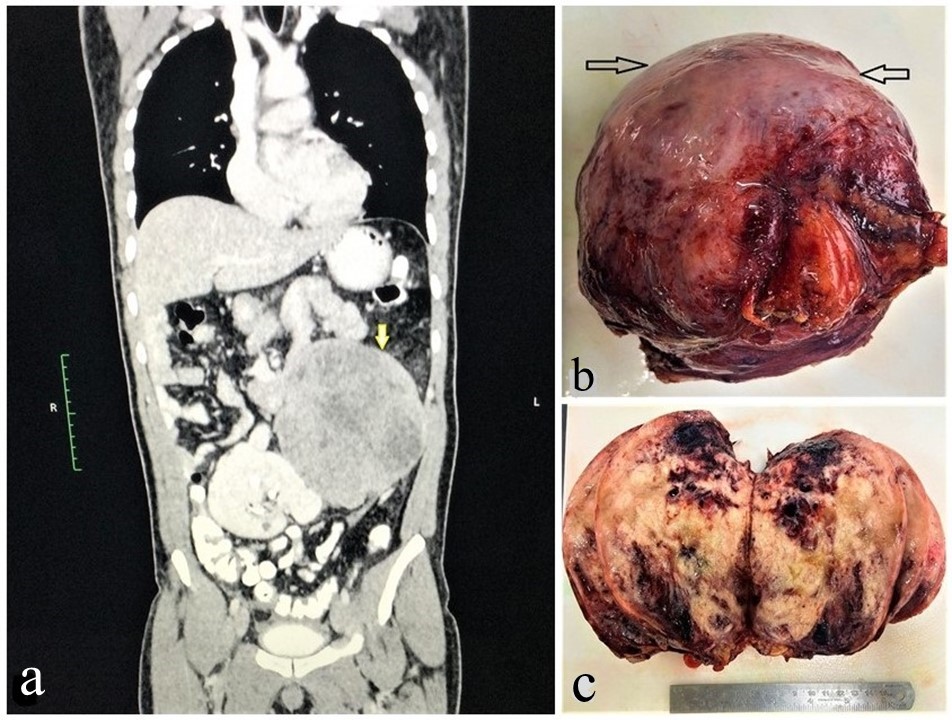
Figure 1: (a) CECT Abdomen showing a heterogeneously enhancing mass (indicated by yellow arrow) attached with the bowel loops, (b) Grossly, specimen is well circumscribed and covered with glistening mesentery (black arrow), (c) Cut surface shows solid grey white to tan color with areas of hemorrhage.
Microscopy revealed proliferation of small round cells in nests, sheets encircling Pacinian corpuscle of the mesentery, in a fibro-myxoid stroma (Figure 2a). The cells had scant cytoplasm, round to oval nuclei and inconspicuous nucleoli (Figure 2b). Tumor cells expressed membranous CD99 (CD, cluster of differentiation) (Figure 2c), friend leukemia virus integration-1 (FLI1) and NK2 homeobox 2 (NKX2.2) (Figure 2d). Anion exchanger 1 (AE1), synaptophysin, chromogranin, desmin, CD117, Wilm’s tumor 1(WT1), CD34, terminal deoxynucleotidyl transferase (TdT), transducin like enhancer of split 1(TLE1) were all negative.
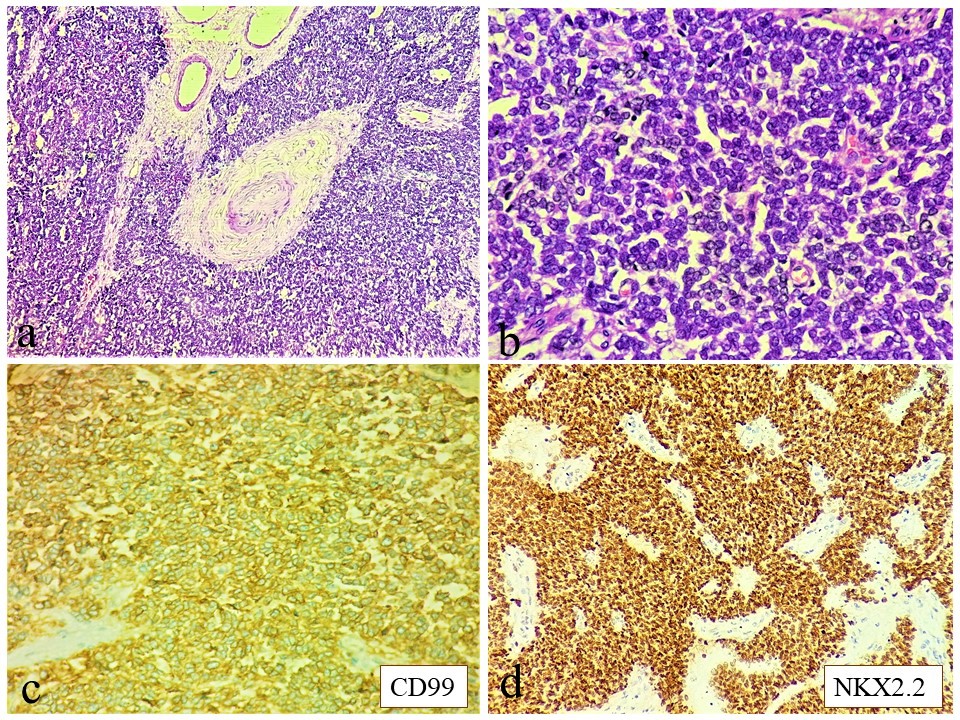
Figure 2: Microscopy (a): Sheets of small round blue cells encircling a Pacinian corpuscle of the mesentery (H&E 40x); (b) Tumor cells having scanty cytoplasm, round to oval nuclei with inconspicuous nucleoli (H&E 400x); (c&d) Tumor cells express CD99 (membranous) & NKX2.2 respectively (IHC 400x); H&E hematoxylin & eosin IHC immunohistochemistry.
Corroborating histomorphology with specific IHC biomarker expression, a diagnosis of extra-skeletal Ewing sarcoma was rendered and molecular study was not done. Bone marrow was uninvolved. Till date, the patient received 6 cycles of adjuvant chemotherapy. The regimen consisted of 3 cycles of vincristine, doxorubicin and cyclophosphamide alternating with 3 cycles of ifosfamide and etoposide. He has no local recurrence or distant metastasis in 6-month follow up period.
Discussion
Extra-skeletal ES is rare and confers a poor prognosis [4]. The most common primary sites are in the lower extremities (41%), followed by pelvis (26%), chest wall (16%), upper extremities (9%), spine (6%), hands and feet (3%) and skull (2%) (12). Mesentery around intestine is an extremely rare site. Till date, to the best of our knowledge, nearly 36 cases of intestinal ES have been documented in the literature [2]. Clinically it is characterized by rapid growth of the soft tissue mass, often being manifested as early metastasis to lung, lymph nodes, and bone [4].
The imaging features of extra-skeletal ES are non-specific. Computed tomography (CT) reveals a large, well-circumscribed mass, relatively hypodense or isodense compared to the adjacent muscle. Intra-tumoral necrosis having lower attenuation, confers a heterogeneous enhancement. On magnetic resonance imaging (MRI), T1-weighted images show low to intermediate signal intensity and T2-weighed images exhibits high signal intensity with heterogeneous contrast enhancement [5].
Extra-skeletal ES poses significant challenges in pathology in unusual location. On histopathology, the most common differential diagnoses are other malignant small round cell tumors within the abdominal cavity. Immunohistochemistry helps to establish a correct diagnosis excluding others. The list of differential diagnoses along with expected immunoprofile has been provided in Table 1. A combination of CD99 and FLI1 is the most sensitive and specific test panel for the diagnosis of ES [6]. The NKX2.2 gene, a target of EWS-FLI1, is a recently described useful marker with a sensitivity of 93% and a specificity of 89% [7].
Table 1: Differential diagnoses of intra-abdominal malignant small round cell tumors.
|
Tumor type
|
Immunohistochemical biomarkers
|
|
AE1
|
CD99
|
Desmin
|
Syn
|
TLE1
|
WT1
|
NKX2.2
|
DD1T3
|
TdT
|
|
Ewing sarcoma
|
+ (f)
|
+
|
-
|
-
|
-
|
-
|
+
|
-
|
-
|
|
Desmoplastic small round cell tumor
|
+
|
-
|
+
|
+
|
-
|
-
|
-
|
-
|
-
|
|
Synovial sarcoma (poorly differentiated)
|
+
|
+/-
|
-
|
-
|
+
|
-
|
-
|
-
|
-
|
|
High grade myxoid /round cell liposarcoma
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
|
Wilm’s tumor
|
+ (e)
|
-
|
+(m)/-
|
-
|
-
|
+
|
-
|
-
|
-
|
|
Neuroblastoma
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
-
|
-
|
|
Lymphoblastic Lymphoma
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
Note: + Positive; - Negative; f- focal; +/- usually positive; e- Epithelial component; m- Positive in myogenic differentiation; AE1- Anion exchanger 1; CD- Cluster of differentiation; Syn- Synaptophysin; TLE1- Transducin like enhancer of split 1; WT1- Wilm’s tumor 1; NKX2.2- NK2 Homeobox 2; DDIT3-DNA damage-inducible transcript 3; TdT- Terminal deoxynucleotidyl transferase.
Extra-skeletal ES is treated in a same way as its skeletal counterpart. The treatment consists of surgery, chemotherapy, and radiotherapy. The 5-year survival rate after surgery and chemotherapy is approximately 70%. Prognosis is poor in older individuals [8].
Conclusion
Extra-skeletal Ewing sarcoma can even occur primarily from the mesentery. Exclusion of other differential diagnoses in intraabdominal location by histopathology and immunohistochemistry are of utmost necessity for proper management and to reduce the risk of recurrence or metastasis.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Folpe AL, Hill CE, Parham DM, O'Shea PA, Weiss SW. Immunohistochemical detection of FLI-1 protein expression: A study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing's sarcoma/primitive neuroectodermal tumor. Am J Surg Pathol. 2000; 24(12):1657–1662.
[2] Yang J, Wei H, Lin Y, Lin N, Wu S et al. Challenges of Diagnosing Primary Ewing’s Sarcoma in the Small Intestine of the Elderly: A Case Report. Front Oncol. 2021; 11:565196.
[3] Peng L, Yang L, Wu N, Wu BO. Primary primitive neuroectodermal tumor arising in the mesentery and ileocecum: A report of three cases and review of the literature. Exp Ther Med. 2015; 9(4):1299–1303.
[4] Jiang S, Wang G, Chen J, Dong Y. Comparison of clinical features and outcomes in patients with extraskeletal vs skeletal Ewing sarcoma: an SEER database analysis of 3,178 cases. Cancer Manag Res. 2018; 10:6227–6236.
[5] Revannagowda S, Gangadhar K, Akaike G, Dighe M. Primary Intra-Abdominal Ewing’s Sarcoma in Adults: a Multimodality Imaging Spectrum. Curr Probl Diagn Radiol. 2020; 49(2):133–139.
[6] Mhawech-Fauceglia P, Herrmann F, Penetrante R, Beck A, Sait S et al. Diagnostic utility of FLI-1 monoclonal antibody and dual-colour, break-apart probe fluorescence in situ (FISH) analysis in Ewing's sarcoma/primitive neuroectodermal tumour (EWS/PNET): A comparative study with CD99 and FLI-1 polyclonal antibodies. Histopathology. 2006; 49(6):569–575.
[7] Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K et al. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012; 36(7):993–999.
[8] Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A et al. Ewing’s sarcoma family of tumors: Current management. Oncologist. 2006; 11(5):503–519.