Original Research
2018
March
Volume : 6
Issue : 1
Antibiotic susceptibility pattern of Burkholderia cepacia complex from various clinical samples in a tertiary care center: A one year prospective study
Ratna Shukla, Anil K Bilolikar, Udayasri B, Pragya Rani
Pdf Page Numbers :- 1-5
Ratna Shukla1,*, Anil K Bilolikar1, Udayasri B1 and Pragya Rani1
1Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. Ratna Shukla, Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Email: ratnashukla8@gmail.com
Received 05 October 2017; Revised 17 November 2017; Accepted 30 November 2017; Published 08 December 2017
Citation: Shukla R, Bilolikar AK, Udayasri B, Pragya Rani. Antibiotic susceptibility pattern of Burkholderia cepacia complex from various clinical samples in a tertiary care center: A one year prospective study. J Med Sci Res. 2018; 6(1):1-5. DOI: http://dx.doi.org/10.17727/JMSR.2018/6-1
Copyright: © 2018 Shukla R et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The Burkholderia cepacia complex (BCC) is a diverse group of human pathogens that cause life-threatening infections in patients with indwelling devices & those requiring intensive care. The aim of this study is to determine the antibiotic susceptibility pattern of Burkholderia cepacia complex (BCC) from various clinical samples in our hospital.
Material and methods: In this one year prospective study conducted in the Department of Microbiology at Krishna Institute of Medical Sciences Limited, Secunderabad; various samples received over the year were cultured. Identification and antibiotic susceptibility testing of BCC was done by using automated Vitek® 2 Compact system (BioMérieux).
Results: Out of total 4900 culture positive isolates; 43 (0.9%) Burkholderia cepacia complexes were isolated. Majority isolates were from blood 18 (41.8%) followed by respiratory samples 15 (34.8%). BCC showed maximum susceptibility to ceftazidime (72.1%) and minocycline (55.8%). Maximum resistance was seen with β–lactamase inhibitor drugs (83.7%).
Conclusion: BCC being a nosocomial agent with high mortality poses a real threat in critically ill patients and needs prompt treatment and infection control.
Keywords: Burkholderia cepacia complex; multi-drug resistance; Vitek 2
Full Text
Introduction
Burkholderia cepacia is a cluster of at least 18 closely related genomic species (or genomovars) now called the Burkholderia cepacia complex (BCC) (Table 1). Since early 1980’s, Burkholderiacepacia has emerged as a cause of opportunistic human infections. The Burkholderia cepacia complex is a group of oxidase positive, non-lactose fermenting Gram negative bacilli having a spectrum of infections ranging from superficial to deep seated and disseminated infections. BCC is frequently associated with epidemic spread and with “Cepacia syndrome” which is manifested by severe progressive respiratory failure and bacteremia. Virulence markers such as cable (cbl) pilus encoded by cable pilin subunit gene (cblA) mediates adherence to mucus glycoproteins and enhances adherence to epithelial cells. Burkholderia epidemic strain marker (BCESM) associated with Burkholderia cepacia strain types infects multiple patients with cystic fibrosis; occurs exclusively in Burkholderia cenocepacia [1].
Table 1: Burkholderia cepacia complex (BCC).
|
1. Burkholderia cepacia
|
10. Burkholderia arboris
|
|
2. Burkholderia multivorans
|
11. Burkholderia contaminans
|
|
3. Burkholderia cenocepacia
|
12. Burkholderia diffusa
|
|
4. Burkholderia stabilis
|
13. Burkholderia lata
|
|
5. Burkholderia vietnamiensis
|
14. Burkholderia latens
|
|
6. Burkholderia dolosa
|
15. Burkholderia metallica
|
|
7. Burkholderia ambifaria
|
16. Burkholderia pseudomultivorans
|
|
8. Burkholderia anthina
|
17. Burkholderia seminalis
|
|
9. Burkholderia pyrrocinia
|
18. Burkholderia ubonensis
|
BCC are commonly found on plant roots, soil and moist environments [2]. BCC strains are transmissible between patients and that cross-infection occurs by direct person-to-person spread. It has been isolated from numerous water sources and wet surfaces; including detergent solutions and IV fluids [3, 4]. It has emerged as a serious nosocomial pathogen worldwide especially in patients with indwelling devices. With the limited recommended antibiotic options available for BCC, emerging antibiotic resistance is of great concern.
Material and methods
This is a one year prospective study conducted in the Department of Microbiology at Krishna Institute of Medical Sciences Limited, Secunderabad. The study period is from September 2016 to August 2017. In this study; various clinical samples like blood, sputum and other respiratory samples, urine, pus, fluids, etc. from intensive care units, Organ transplantation units, outpatient department and wards submitted to Microbiology Laboratory at KIMS hospital, Secunderabad were collected. Samples of new born and pregnant women were excluded from this study. They were processed and cultured based on standard microbiological guidelines. Primary identification was done by examining Gram’s stained smears and colony morphology on culture media. The final identification of BCC and its antibiotic susceptibility testing was done using GN and AST 281 cards, respectively in automated Vitek® 2 Compact system (BioMérieux) [5]. The quality control for GN card was done by using ATCC700323– Enterobacter hormaechei, ATCC17666- Stenotrophomonas maltophilia. The quality control for AST N281 card was done by using ATCC25922 – Escherichia coli, ATCC 27853- Pseudomonas aeruginosa and ATCC 35218- Escherichia coli, as per manufacturer’s instruction. Minimum inhibitory concentration (MIC) values of antibiotics were obtained and reporting was done as per the CLSI guidelines 2016&2017 [6, 7]. The data was captured from the system and analyzed.
Vitek® 2 Compact system
It is an automated microbiology system utilizing growth-based technology. It makes use of colorimetric reagent cards that are incubated and interpreted automatically. It has application in clinical laboratories. It is also compliant for electronic records & signatures. A colorimetric reagent card GN is used for identification of Gram negative bacteria.

Figure 1: Vitek 2 Compact system.
Results
Out of total 4900 culture positive isolates over the year; 43 (0.9%) Burkholderiacepacia complexes were isolated. BCC were isolated from blood, endotracheal secretion, sputum, bronchial wash, urine and other samples (pus, pigtail fluid, dialysis tip, etc.). Maximum isolates were from blood - 18 (41.8%) followed by respiratory samples – 15 (29%) shown in Table 2. Figure 2 shows that majority isolates were from ICU’s - 25 (58%) (Table 3) followed by wards 13 (30%). Table 4 shows major group of patients belonged to median age group between 30-60 years. BCC isolates were common in males as compared to females. BCC showed maximum susceptibility to ceftazidime (72.1%) and minocycline (55.8%) (Figure 3). Maximum resistance was seen with ticarcillin-clavulanic acid (83.7%) combination drug. Figure 4 shows month-wise distribution of BCC isolates over the year.
Table 2: Samples with BCC isolates.
|
Samples type
|
Culture positive (43)
|
|
Number
|
%
|
|
Blood
|
18
|
41.8
|
|
ET secretion
|
7
|
16.2
|
|
Sputum
|
5
|
11.6
|
|
Bronchial wash
|
3
|
6.9
|
|
Urine
|
2
|
4.6
|
|
Others
|
8
|
18.6
|
|
|
|
|
Table 3: Distribution of BCC isolates in inpatients and outpatients.
|
Cases
|
Number of Isolates
|
Percentage
|
|
IP
|
ICU’s
|
25
|
58%
|
|
WARDS
|
13
|
30%
|
|
OP
|
5
|
12%
|
Table 4: Age-wise distribution of BCC.
|
Age group
|
Number of BCC Isolates
|
|
≤ 1 month
|
2
|
|
1 month - 12 years
|
4
|
|
13 years - 30 years
|
4
|
|
31 years - 60 years
|
18
|
|
> 60 years
|
15
|
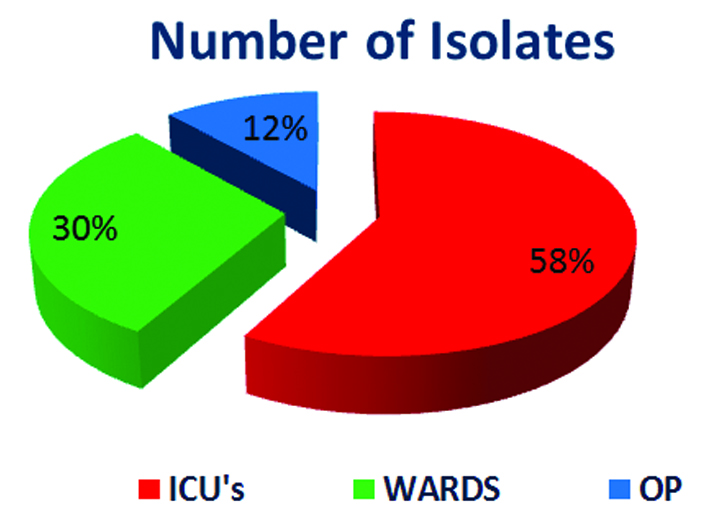
Figure 2: Percentages of isolates.
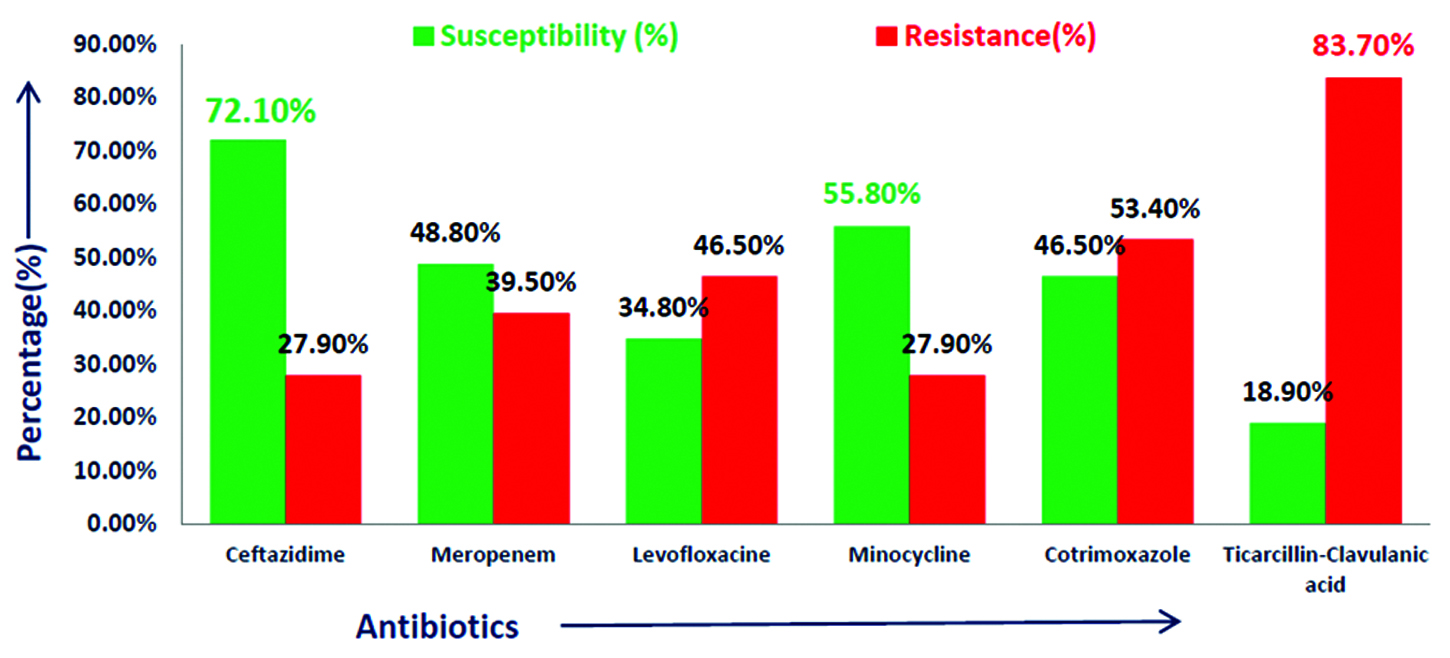
Figure 3: Antimicrobial susceptibility pattern of Burkholderia cepacia complex.
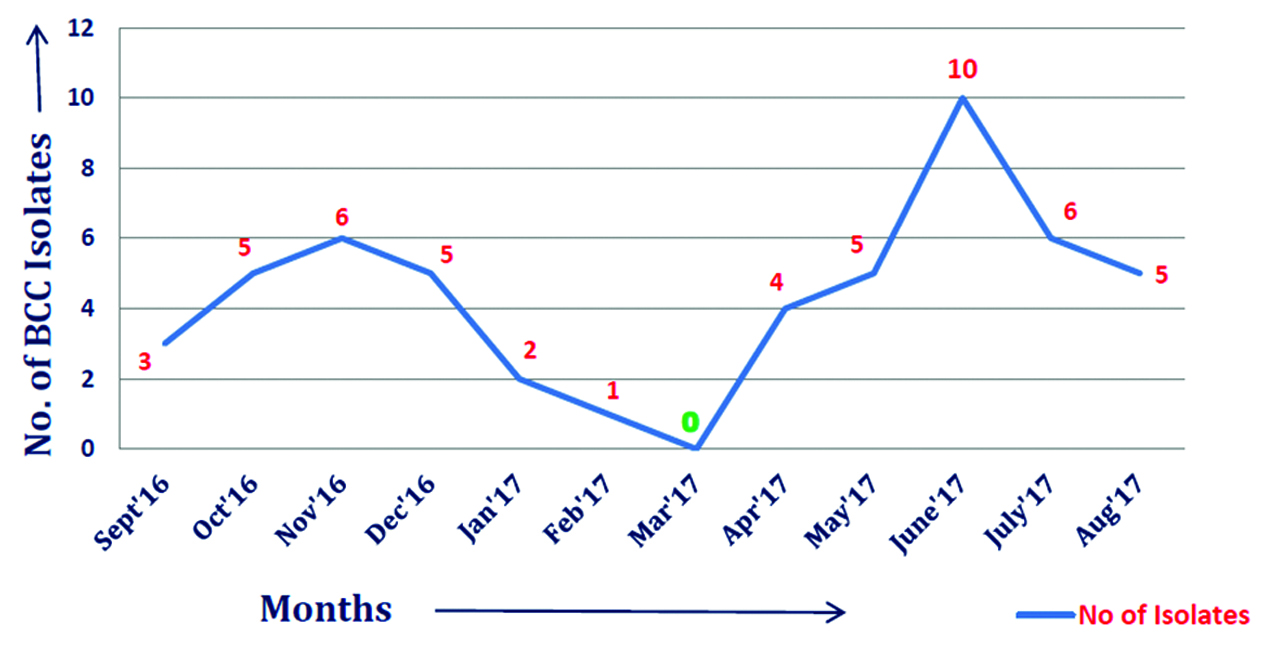
Figure 4: Monthly Burkholderia cepacia complex isolates distribution from September 2016-August 2017.
Discussion
Burkholderia cepacia complex has emerged as an important cause of morbidity and mortality in hospitalized patients. BCC shows intrinsic resistance for many antibiotics. As per Clinical and Laboratory Standards Institutes guidelines 2016–2017, recommended drugs for BCC are ceftazidime, meropenem, minocycline, levofloxacin, cotrimoxazole and ticarcillin-clavulanic acid [6, 7]. However, recently resistance to these drugs is on rise. In this study maximum susceptibility of BCC was seen with ceftazidime which is comparable with study conducted by Dutta et al. [8]. In a five year study by Bhavana MV et al. maximum susceptibility was seen with Cotrimoxazole [9]. Majority isolates were from ICU’s in this study which is also seen in the study conducted by Dizbay et al. [10].
Recently a new drug combination ceftolozane-tazobactam has been added to the list of treating drugs for complicated BCC infection. In a study conducted by Dale et al., ceftolozane-tazobactam demonstrated marginally superior activity over that of ceftazidime against ceftazidime-susceptible strains and retained activity against most (60%) multidrug resistant and extensively drug-resistant strains [11]. In another study conducted by Omar et al., another combination drug moxifloxacin and ceftazidime showed synergistic effect for Burkholderia infection [12]. Further studies and guidelines are needed for practical application and use of such drugs in practice.
With the emerging resistance to even the minimum available recommended drug choices as per CLSI guidelines; there is need for emphasis on rational use of antibiotics and prompt treatment of BCC infections. This study outlines the susceptibility data that can help in making empirical choice and brings in the need for more research in this field.
The limitation of this study is lack of molecular confirmation due to economic constraints. It is a laboratory based study so outcome could not be measured. However, this study signifies the need of identification of BCC on routine basis as it has emerged as a significant nosocomial pathogen and can be a cause of hospital outbreaks. It amounts to morbidity and mortality increasing the cost of hospital stay and loss of life. With intrinsic resistance to various antibiotics and emergence of resistance to minimum available antibiotics, outbreaks with such strains being preventable in hospitals; needs to be kept check on. Continuous monitoring is important in this case to prevent any such outbreak.
Conclusion
Burkholderia cepacia complex being a nosocomial agent with high mortality poses a real threat in critically ill patients and needs prompt treatment. Hospital infection control committee and antibiotic stewardship committee plays a major role in preventing these infections. Acknowledgement The Department of Microbiology, KIMS Hospital, Secunderabad, for giving me the opportunity to conduct this study.
Conflict of interest
Authors declare no conflict of interest.
References
[1] Koneman EW, Allen SD, Janda WM, Winn WC, Schreckenberger PC, et al. Koneman’s Color atlas and textbook of diagnostic microbiology. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2006.
[2] Gautam V, Singhal L, Ray P. Burkholderia cepacia complex: Beyond Pseudomonas and Acinetobacter. Indian J Med Microbiol. 2011; 29(1):4–12.
[3] Mali S, Dash L, Gautam V, Shastri J, Kumar S. An outbreak of Burkholderia cepacia complex in paediatric unit of a tertiary care hospital. Indian J Med Microbiol. 2017; 35:216–220.
[4] De Smet B, Veng C, Kruy L, Kham C, van Griensven J, et al. Outbreak of Burkholderia cepacia bloodstream infections traced to the use of Ringer lactate solution as multiple-dose vial for catheter flushing, Phnom Penh, Cambodia. Clin Microbiol Infect. 2013; 19(9):832–837.
[5] Pincus DH. Microbial identification using the bioMérieux VITEK® 2 system. Encyclopedia of Rapid Microbiological Methods. 2010; 1–32.
[6] Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 26th informational supplement M100-S26. Wayne, USA: CLSI; 2016.
[7] Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement M100-S27. Wayne, USA: CLSI; 2017.
[8] Dutta S, Haq S, Hasan MR, Haq JA. Antimicrobial susceptibility pattern of clinical isolates of Burkholderia pseudomallei in Bangladesh. BMC Res Notes. 2017; 10(1):1–5.
[9] Bhavana MV, Joshi S, Adhikary R, Beena HB. Antibiotic susceptibility pattern of Burkholderia cepacia complex and Stenotrophomonas maltophilia: A 5-year analysis. Indian J Med Microbiol. 2017; 35(2):318–319.
[10] Dizbay M, Guzel O, Ergut B, Aktas F, Arman D. Nosocomial Burkholderia cepacia infections in a Turkish university hospital: a five-year surveillance. J Infecr Dev Ctries. 2009; 3(4):273–277.
[11] Dale MM, Carol Y, M KL, Theodore S, Li Puma JJ. In Vitro Activity of ceftolozane- tazobactam and other antimicrobial agents against Burkholderia cepacia complex and Burkholderia gladioli. Antimicrob Agents Chemother. 2017; 61(9):e00766–17.
[12] El-halfawy OM, Naguib MM, Valvano MA. Novel antibiotic combinations proposed for treatment of Burkholderia cepacia complex infections. Antimicrob Resist Infect Control. 2017; 6:120.