Full Text
Introduction
A cardiac arrhythmia is characterised by an irregular heart rhythm — the consequence of an abnormality in the site of the origin of impulse, rate, or conduction. There are two types of arrhythmias: bradyarrhythmia and tachyarrhythmia. Bradyarrhythmia occurs in patients with a heart rate of less than 60 beats per minute (bpm), whereas tachyarrhythmia occurs in patients with a heart rate of more than 100 bpm.
An electrocardiogram (ECG) is the first investigation ordered for evaluation and most of the time is the most important assessment tool for arrhythmias. Sometimes arrhythmias may be happening infrequently or silently (e.g., AF) and in that condition event loop recorders (ELRs) or implantable loop recorders (ILRs) may prove useful in establishing the diagnosis. And ultimately in a large group of patients an electrophysiological study (EPS) is needed to confirm the diagnosis and plan further management. Before we discuss the actual management of various arrhythmias, it is important to discuss the different arrhythmias briefly.
Types of arrhythmias
Bradyarrhythmia
Bradyarrhythmia can result from a disease in the sinoatrial (SA) node or the atrioventricular (AV) node and the specialised His-Purkinje conduction system. It can also result from drugs that block sympathetic systems or are vagotonic. Treatment should rarely be prescribed solely on the basis of a heart rate lower than an arbitrary cutoff or a pause above certain duration. Assessment of symptoms is a critical component in the evaluation and management of bradyarrhythmia.
Sinus bradycardia
In sinus bradycardia, the heart rate is less than 60 bpm and regular. Every P wave is followed by a QRS complex. Mostly sinus bradycardia is benign, and may be a result of high baseline vagal tone — as is seen in well-trained athletes. It can also be the effect of drugs that block the sympathetic system (eg beta blockers). When it is associated with chronotropic incompetence, then it is pathological and a manifestation of sick sinus syndrome.
One common cause for nocturnal bradyarrhythmia is sleep-disordered breathing. The 2018 Bradycardia Guideline gives a class I recommendation for the screening of sleep apnea in patients with nocturnal bradycardia. Patients who were screened positive were considered for sleep studies and speciality consultation. The prevalence of sinus bradycardia in patients with sleep apnea can be as high as 40%, with episodes of second-or third-degree AV block in up to 13% of patients. Treatment of underlying sleep apnea can result in a near 90% reduction in bradycardia events. Patients with nocturnal bradycardia, not associated with symptoms, almost never require permanent pacing [2, 3].
Sinus exit blocks/pause
The prevalence of sinus-node dysfunction has been estimated to be as high as 1 in 600 patients over the age of 65 years, and the syndrome accounts for approximately 50 percent of pacemaker implantations worldwide. Sinus exit blocks are a manifestation of an intrinsic sinus node disease. This often occurs because of two reasons. The first is that the sinus node fails to discharge automatic impulses. The second reason is that there is a block in the transmission of impulse into the surrounding atrial tissue.
A sinus exit block may be classified under three degrees. First degree sinus exit block is characterized by a sinus node impulse that is delayed in reaching the surrounding atrium. While it can sometimes be associated with a visible PR prolongation, such a block cannot be easily diagnosed on an ECG. Only an electrophysiology (EP) study can detect a delay in impulse to travel from SA node to the surrounding atrium. In second degree sinus exit block, there is an intermittent failure of the SA node impulse in reaching the surrounding atrium. This can be diagnosed on an ECG by the presence of intermittent absent P waves. Third degree sinus exit block occurs when there is a complete cessation of transmission. It can only be diagnosed on ECGs when the P waves are absent for variable periods. Third degree SA exit blocks and sinus node arrest are impossible to differentiate on ECG, so it is better to term them as sinus pause (Figure 1).
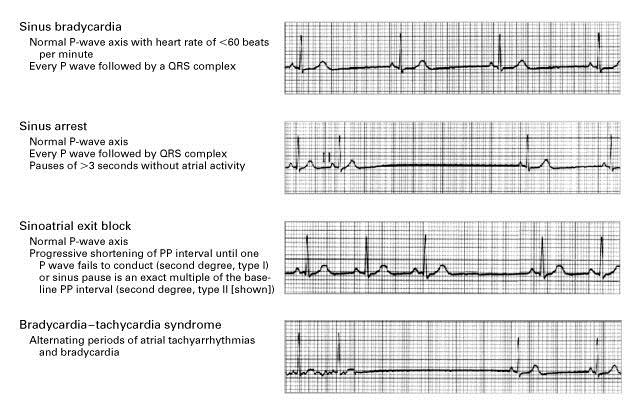
Figure 1: Sick sinus syndrome.
Atrioventricular (AV) blocks
The atria and ventricles are electrically insulated from each other. As a result, the AV node-his bundle system forms the only channel for any impulse to travel in between the upper and lower chambers of the heart. A block in the impulse transmission in the AV node-his bundle system is referred to as an AV block. Such blocks are also classified under three degrees. First degree AV block occurs when there is a delay in impulse conduction. On an ECG, this is defined as a PR duration of more than 200 milliseconds. Second degree AV block is characterised by intermittent failure of the P wave to conduct through the AV node system. This AV block is further classified as Mobitz type 1 and Mobitz type 2. In Mobitz type 1 second degree AV block, there is progressive prolongation of the PR interval, followed by a non-conducted P wave — also known as Wenckebach phenomenon. In Mobitz type 2 second degree AV block, intermittent P waves are not followed by QRS complexes. Additionally, the PR interval does not vary before the dropped beat. Third degree AV block occurs when there is a complete failure of P waves to conduct to the ventricle for variable periods. On an ECG, this manifests as a sequence of P waves without any accompanying QRS waves. Patients with this disease develop syncope, which is called Stokes-Adams syndrome. These patients may gain consciousness with the restoration of circulation as a result of slower escape rhythm either from the AV junction or his/infrahisian region — depending upon the level of block. In this situation, there is AV dissociation on the ECG with slow ventricular rates (Figure 2).
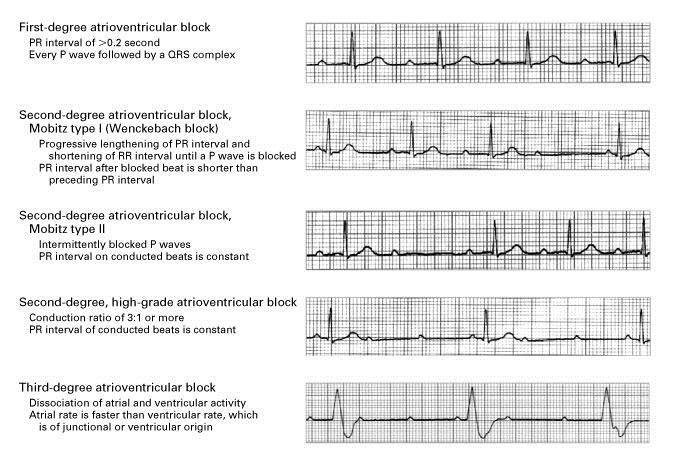
Figure 2: Types of AV blocks.
Tachyarrhythmias
Tachyarrhythmias occur in patients with a heart rate of at least 100 bpm. It is classified as sustained if it lasts for at least 30 seconds or requires termination by cardioversion. Tachyarrhythmia can result from pathology in the atria, the AV junctional region, or the ventricles. It results from three common mechanisms: enhanced automaticity, re-entry or triggered activity. Enhanced automaticity means increased discharge from myocardial cells with automatic discharge capacity — such as the SA node, atrial cells, AV junction, or His bundle purkinje system. Re-entry involves the establishment of a circuit around scar, or non-excitable, tissue with the circuit having variable conduction properties. Trigger activity can enable a cell incapable of spontaneous discharge to start discharging impulses. Tachyarrhythmias can be classified as either a supraventricular tachyarrhythmia (SVT) or a ventricular tachyarrhythmia. Further tachyarrhythmias are classified as narrow-complex tachycardias (QRS duration ≤ 120 msec), and wide-complex tachycardias (QRS duration >120 msec) [4]. In most instance a narrow-complex tachycardia almost always makes the diagnosis of SVT, a WCT can be supraventricular or ventricular. Narrow-complex tachycardia can occur in VT if it arises near the his-purkinje system (Septum) or a VT can be paradoxically narrower if baseline QRS wide.
Supraventricular tachyarrhythmias
Supraventricular tachyarrhythmia refers to any arrhythmia arising in or involving the atrium as part of the electrical circuit. This includes sinus tachycardia, sinus node reentry tachycardia, atrial tachycardia (AT), AFl, AF, and junctional tachycardia [5].
Sinus tachycardia results from enhanced automaticity of the sinus node, either secondary to sympathetic stimulation or due to inappropriate sinus tachycardia. Sinus node reentry tachycardia and sinus tachycardia are difficult to differentiate on ECG since the P wave morphology in sinus node reentry tachycardia is similar to the sinus rhythm. In sinus tachycardia, the heart rate is usually no greater than 180 bpm. AT can result from enhanced automaticity or micro-reentry in the atrial tissue [6]. The morphologies of the P wave and the PR interval depend upon the site of origin and the atrial size. The atrial rate in AT is usually not more than 200 bpm. At lower rates, there may be 1:1 AV conduction. However, at higher rates — or under vagotonic influence — it is possible that there may not be 1:1 AV conduction (with the ventricular rate being less than the atrial rate) (Figure 3).
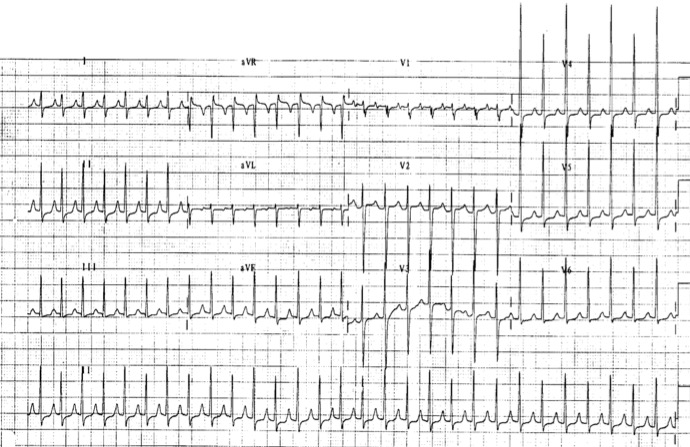
Figure 3: Atrial tachycardia; surface ECG shows a P wave preceding each QRS complex, although at rapid heart rates, the P wave may be obscured by the T wave.
AFl is an example of macro-reentry tachycardia involving the atrium. Typical AFl involves the Cavo-tricuspid isthmus (CTI) and may be clockwise or counterclockwise. The AFl is labeled as clockwise when the impulse comes down the atrial septum and passes through the CTI before ascending over the crista terminals to reach the upper atrial septum. If it is in the opposite direction, it is called counterclockwise. On the ECG, the AFl is characterised by sawtooth P waves. In clockwise flutter, the P wave is positive in inferior leads and negative in V1 [7]. In counterclockwise flutter, however, the P wave is negative in inferior leads and positive in V1 (Figure 4). Sometimes, the flutter may arise around a scar area — leading to variations in the P-wave morphology that are distinct from those mentioned above. This is called atypical flutter [8]. In AFl, the atrial rate ranges from 250-350 bpm, and the AV relation may be 1:1 — fixed or variable.
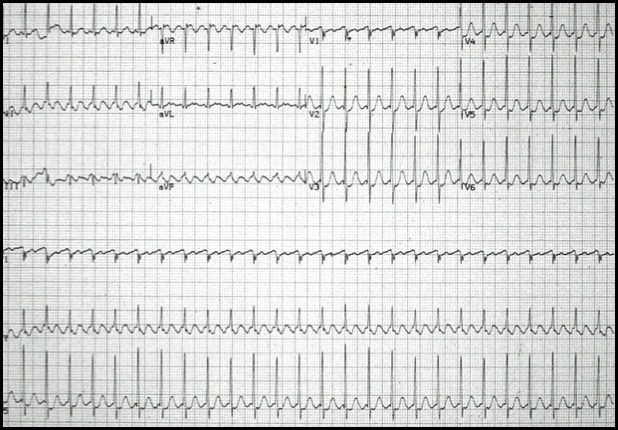
Figure 4: Atrial flutter counter clockwise (CCW), isthmus dependant, negative flutter waves in II, III, aVF.
AF results from fast, chaotic atrial discharges — often arising around the pulmonary vein ostia in the left atrium [9]. The atrial rate is usually greater than 300 bpm and is characterized by variable AV conduction. On an ECG, it manifests as fibrillatory waves (non-discernible P waves) and variable RR intervals. The RR interval becomes fixed only when there is an underlying complete AV block (Figure 5).
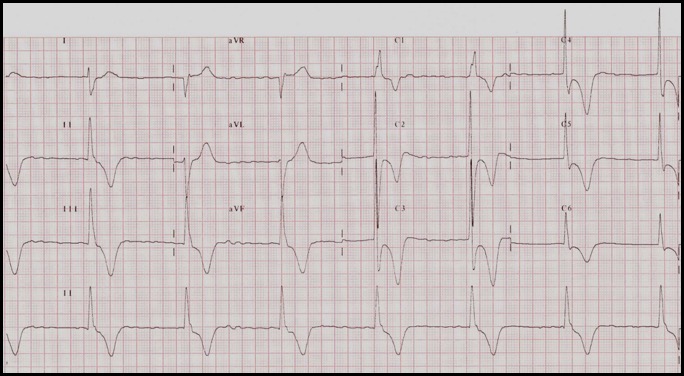
Figure 5: AF with slow and fixed ventricular rate suggestive of underlying complete heart block.
Junctional tachycardia can be either non-reentrant or reentrant. Non-reentrant junctional tachycardia is characterised by enhanced automaticity or triggered activity from the AV junctional region — resulting in tachycardia with QRS morphology similar to the sinus rhythm. It is important to note that there may or may not be retrograde P waves accompanying the QRS complex on an ECG. This type of junctional tachycardia is seen in infants and called paroxysmal junctional reentrant tachycardia (PJRT) and post-cardiac surgery patients — among others.
Reentrant junctional tachycardia results from reentry involving the AV node’s junctional tissue. It is commonly referred to as paroxysmal supraventricular tachycardia (PSVT). If the reentry happens at the level of the AV node, it is termed AV nodal reentrant tachycardia (AVNRT) (Figure 6). In typical AVNRT, the antegrade limb is formed by the slow pathway while the retrograde limb is formed by the fast pathway — resulting in a very short VA interval (less than 80 milliseconds). In atypical AVNRT, the retrograde limb is formed by the slow pathway, and the VA interval is prolonged. When the reentry involves an accessory pathway between the atrium and ventricle — and the other limb of the circuit is formed by the AV node-His bundle system — it is referred to as atrio-ventricular reentry tachycardia (AVRT). AVRT may be either orthodromic or antidromic. In orthodromic AVRT, the antegrade limb is through the AV node-His bundle system, while the retrograde limb is via the accessory pathway. The accessory pathway may be left-sided, right-sided, or through the septum. Orthodromic AVRT usually manifests as a narrow QRS tachycardia on an ECG with morphology similar to the baseline QRS, unless there is aberrancy as a result of rate-dependent bundle branch block (BBB) (Figure 7). When the antegrade limb is formed by the accessory pathway and the retrograde limb by the His bundle-AV node, it is termed antidromic AVRT [10]. Antidromic AVRT on an ECG manifests as a wide QRS tachycardia (Figure 8). It notoriously creates a diagnostic dilemma with ventricular tachycardia. Accessory pathways may or may not manifest on an ECG. A manifested accessory pathway on the ECG shows pre-excitation (short PR and slurred delta wave), wide QRS, and secondary T-wave changes. A manifested accessory pathway in a patient exhibiting palpitation is called WPW syndrome (Wolff-Parkinson-White syndrome). A concealed accessory pathway is characterized by absent antegrade conduction property — resulting in a baseline ECG that appears normal.
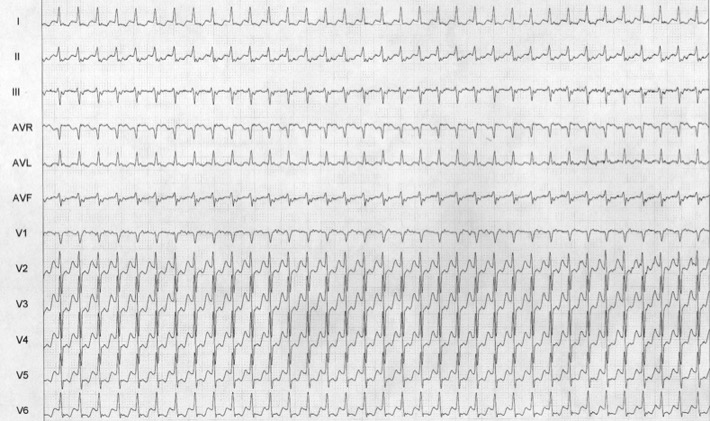
Figure 6: PSVT - AVNRT; Because of the simultaneous depolarization of the atrium and ventricle, P waves are rarely seen on the surface ECG, although atrial depolarization can occasionally be seen as part of the terminal QRS complex in lead V1.
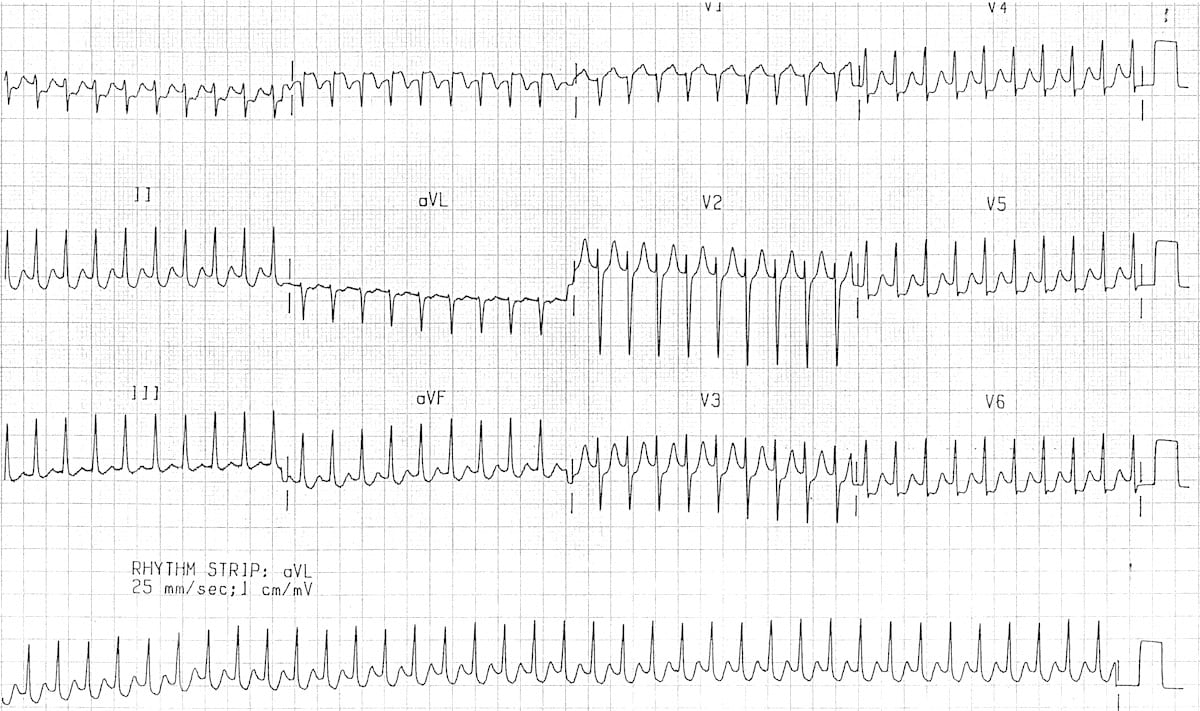
Figure 7: AVRT orthodromic tachycardia - anterograde conduction is via the AV node, producing a regular narrow complex rhythm, long RP tachycardia, retrograde P waves are usually visible.
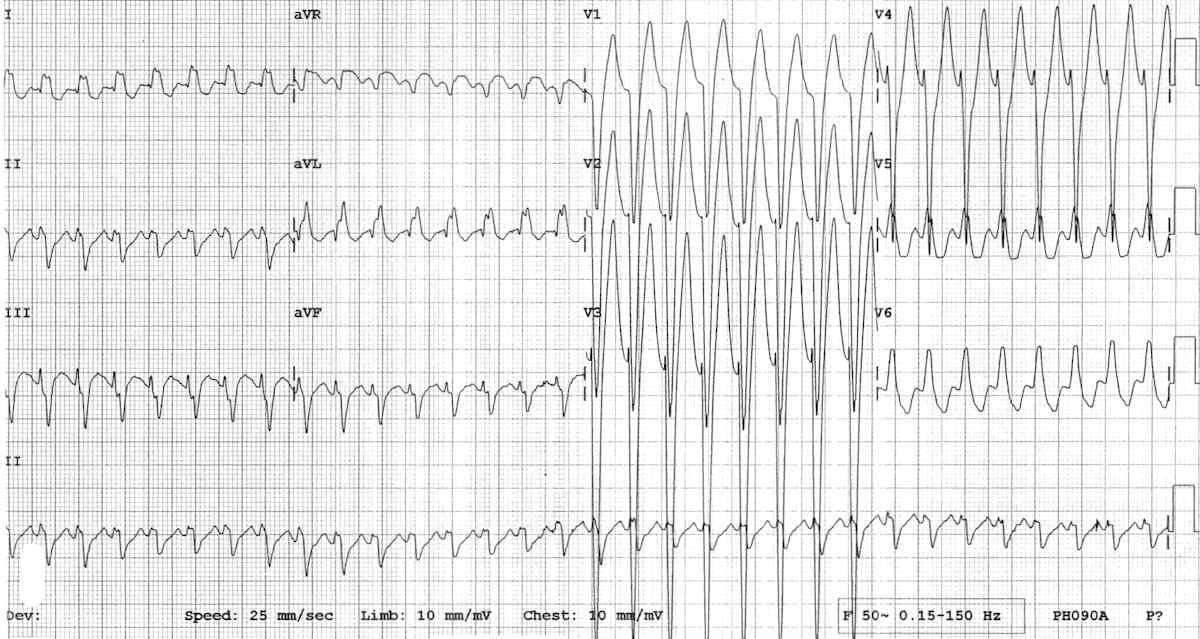
Figure 8: Antidromic tachycardia - anterograde conduction is via the accessory pathway, producing a regular wide complex rhythm. This can be difficult to distinguish from normal heart VT.
Ventricular tachyarrhythmias
Ventricular tachyarrhythmias (VT) can originate either from the right ventricle or the left ventricle. They happen either because of a micro-reentry phenomenon or triggered activity. They are characterised by a well-formed QRS morphology and a heart rate of at least 100 bpm (Figure 9). VT may be monomorphic or polymorphic (multiple QRS morphology). Torsades de pointes is a form of polymorphic VT that occurs with long QT (congenital or acquired) and progressively changing QRS morphology — giving an impression of twisting around the baseline. Slower VT may be hemodynamically stable but fast VT — as well as VT accompanied by LV dysfunction — may result in hemodynamic compromise. VF is always hemodynamically unstable. It is also important to note that when the heart rate exceeds 300 bpm and the ventricular rhythm is irregular and chaotic, ventricular fibrillation (VF) is observed.
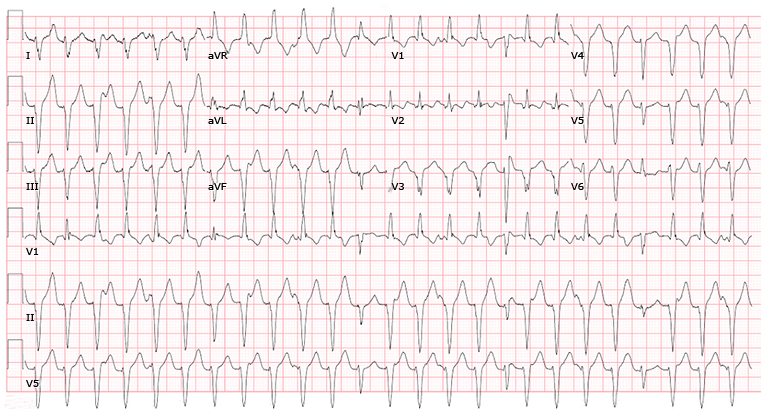
Figure 9: The ECG hallmark for the diagnosis of sustained monomorphic VT is a wide complex tachycardia with the presence of AV dissociation, fusion beats (which reflect a supraventricular impulse coming from above the AV node fusing with an impulse generated in the ventricle), captured beats (which reflect an impulse coming from above the AV node that depolarizes the ventricles when they are no longer refractory but before the next ventricle-generated complex).
Managing arrhythmias
Arrhythmia management is primarily based on the patient’s symptoms and hemodynamic status. Most asymptomatic arrhythmias may be managed by close clinical follow-up. However, symptomatic or hemodynamically significant arrhythmias require definite treatment. The management may be classified as acute and long-term management.
Management of bradyarrhythmia
The most significant symptoms in patients with bradyarrhythmia are presyncope and syncope. A patient presenting with bradycardia and syncope may require a temporary pacemaker insertion [11].
Transcutaneous pacing may be done if the heart rate is less than 40 bpm. I/V Atropine may be given to patients with complete heart block and is effective if the block is suprahisian (narrow QRS escape rhythm) (Table 1).
Table 1: Medication chart.
|
Medication
|
Utility
|
|
Atropine
|
A parasympatholytic drug that can affect sinoatrial conduction, sinus node automaticity, and AV conduction.
|
|
Isoproterenol
Dopamine
Dobutamine
Epinephrine
|
Direct stimulation of beta-receptors to increase sinus node automaticity and AV conduction. Contraindicated in coronary ischemia.
|
|
Intravenous calcium
|
Can be used if bradycardia is attributed to calcium channel blocker overdose.
|
|
Glucagon
|
Can be used if bradycardia is attributed to calcium channel blocker or beta-blocker overdose. May cause nausea and vomiting.
|
|
High-dose insulin
|
Can be used if bradycardia is attributed to calcium channel blocker or beta-blocker overdose. Risk of hypoglycemia.
|
|
Aminophylline
|
Can be used in heart transplant patients, spinal cord injury, or inferior MI with AV block.
|
After the acute management, the patient needs to be evaluated for eligibility for permanent pacemaker implantation (PPI) — provided secondary causes of bradycardia have been ruled out (e.g., acute IWMI, hyperkalemia, drug toxicity). Symptomatic sick sinus syndrome (SSS) is associated with tachyarrhythmia (tachy brady syndrome), which requires anti-arrhythmic drug therapy with a backup of PPI. In patients with complete heart block or high-degree AV block, PPI is needed irrespective of symptoms — especially if the block is infranodal. Patients of AF with high-degree AV block also require PPI. In patients with second degree-morbitz type 1 AV block that is either symptomatic or at the level of His or infrahisian on the electrophysiological study (EPS), then PPI is indicated. However, in patients with first degree heart block, PPI may be considered if PR is greater than 300 milliseconds and the patient is symptomatic [12, 13].
Management of tachyarrhythmias
Symptomatic tachyarrhythmias often present with palpitation, uneasiness, chest discomfort, breathlessness, presyncope, or syncope. Fast tachycardias — particularly VT or VF — present with hemodynamic compromise. The acute management depends upon the hemodynamics of the patient. Any patients with tachyarrhythmia presenting with hemodynamic compromise requires DC cardioversion. A synchronised DC shock is given to treat patients with VT, whereas an unsynchronised DC shock is given to patients with VF (Table 2).
Table 2: Type of arrhythmia and their acute and chronic (Long term) management.
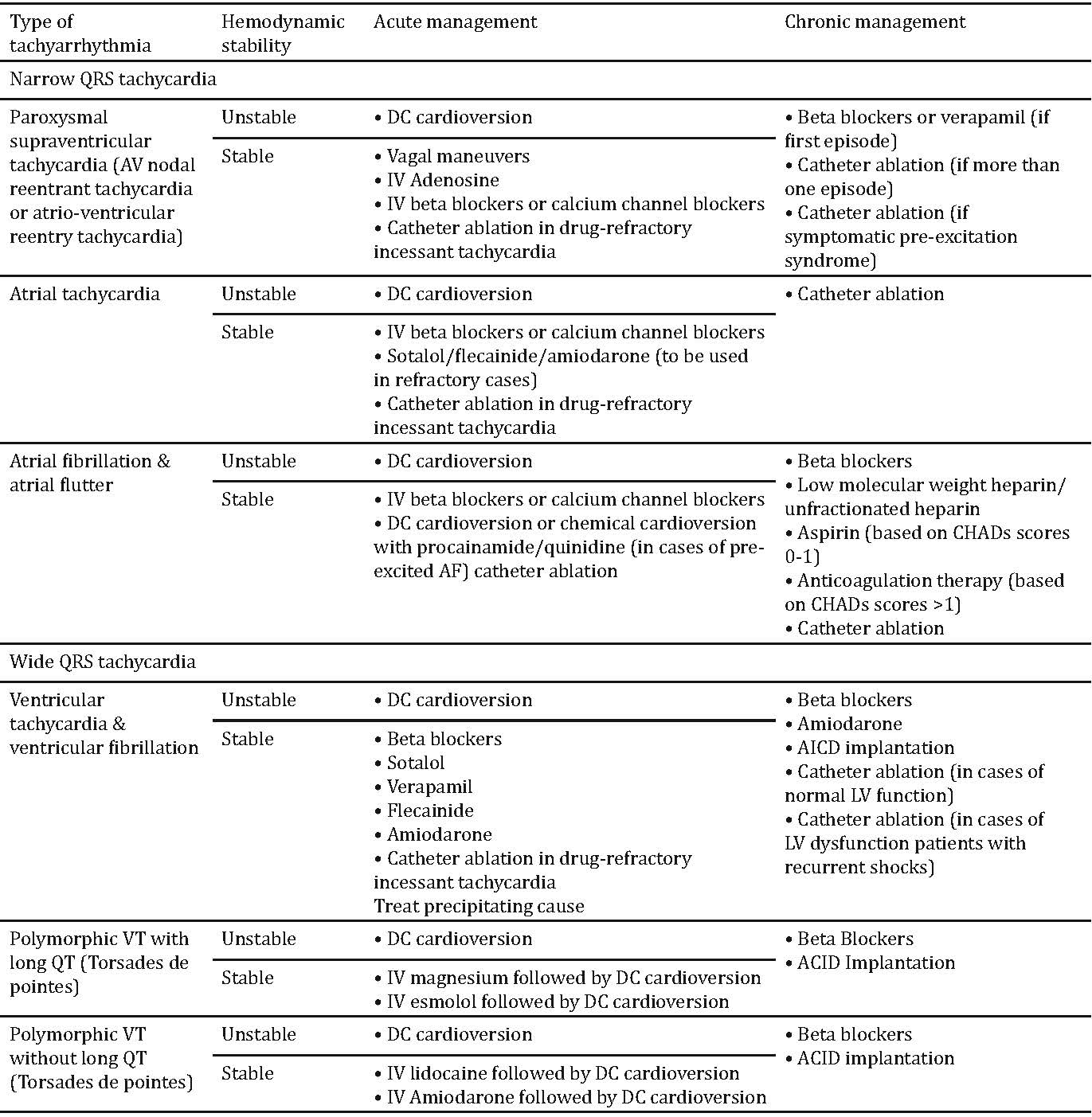
In hemodynamically stable patients, management depends on whether there exists a narrow QRS tachycardia or a wide QRS tachycardia. A narrow QRS tachycardia (QRS duration less than or equal to 120 milliseconds) may be regular or irregular. An irregular narrow QRS tachycardia is most likely caused by AF, but it can also be AT or AFl with variable conduction or multifocal AT. The initial goal in irregular narrow QRS tachycardia should be to control the heart rate using beta blockers or calcium channel blockers. Digoxin may be used in the event that the patient has a low ejection fraction and borderline blood pressure. Intravenous ibutilide has been employed with acceptable success in acute onset AF to restore sinus rhythm. Alternatively, IV amiodarone may be used for acute rhythm control. In AF of less than 48 hours of duration, cardioversion may be done without anticoagulation. However, if the cardioversion is supposed to be done after the 48th hour mark, then left atrium (LA) clots need to be ruled out or anticoagulation drugs need to be given for at least 3 weeks prior to cardioversion.
Chronic management of AF depends on the risk profile and LV function of the patient which also decides the strategy of rhythm control vs rate control in a patient. Based on CHADS2-VASc score, HAS-BLED scores, patients may require a long-term anticoagulation/LA appendage closure device (especially if there is contraindication to anticoagulation/recurrent TIA). Certain patients may benefit from AF ablation therapy (Pulmonary vein isolation) [14].
In a regular narrow QRS tachycardia, the first thing to look for is 1:1, P:QRS relationship (Table 3). If it is a 1:1 conduction, then the RP/PR relationship is assessed. If RP is less than PR, then it is termed a short RP tachycardia. Associated differential diagnoses include AVNRT, AVRT, AT with long PR, sinus node reentrant tachycardia with long PR. If RP is greater than PR, then the following differential diagnoses are possible: atypical AVNRT, AVRT with slow AP, AT and sinus node reentrant tachycardia. If 1:1 P:QRS relationship does not exist and P is greater than QRS, then either an AT or AFl is present. Irrespective of the pathology, initial vagal manoeuvres are useful to induce AV block in narrow QRS regular tachycardia. Such manoeuvres include the carotid sinus massage and the Valsalva manoeuvre (forced expiration against a closed glottis). These techniques may not only act as a diagnostic tool but can also therapeutically differentiate reentrant junctional tachycardias, sinus node reentrant tachycardia and AT. Alternatively, IV adenosine may be employed to produce an AV block [15].
Table 3: Differential diagnosis of supraventricular tachycardias.
|
● The regular SVTs include sinus tachycardia, atrial flutter, AVNRT, AVRT, AT.
● The irregular SVTs are AF, atrial flutter with variable atrioventricular block, and MAT
● Sudden onset and termination are characteristic of acute AF and atrial flutter, AVNRT, AVRT, AT. Gradual onset and recession occur with sinus tachycardia, chronic AF, atrial flutter and MAT.
● I/V adenosine slows the heart rate in sinus tachycardia, AT, AF, or atrial flutter, whereas termination of tachycardia occurs in AVNRT, AVRT, and some ATs.
● I/V Adenosine should not be given in the case of irregular wide-complex tachycardias, since it may render these rhythms unstable.
|
If the arrhythmia terminates after the P wave, a reentrant junctional tachycardia — either an AVNRT or an AVRT — is confirmed. If it terminates after the QRS, then it may be an AVNRT, AVRT, AT or sinus node reentrant tachycardia. An AV node block makes the P waves obvious on the ECG of an AT or AFl -- assisting in the diagnosis of the aforementioned diseases. If vagal manoeuvre and IV adenosine fail to terminate the reentrant junctional tachycardia and the patient is hemodynamically stable, then an IV beta blocker or calcium channel blocker (verapamil or diltiazem) may be given in appropriate doses. After the acute management of reentrant junctional tachycardia, the patient should be offered an EPS and a radiofrequency ablation (RFA) [16]. For certain patients with AT or AFl, RF ablation is advised to treat recurrent symptoms.
A wide QRS regular tachycardia can be VT, SVT with aberrancy, or antidromic AVRT. In patients with ischemic heart disease, LV dysfunction, or preexisting structural heart disease, it is more likely to be VT than any of the other aforementioned diseases. There are various algorithms available to differentiate between VT and SVT in wide QRS tachycardia — such as the Brugada algorithm [17], Vereckei algorithm [18], etc. However, their sensitivity and specificity is only about 80%. The common dictum is that a wide QRS tachycardia should be treated as a VT if there is any doubt. If sustained, the VT needs a DC cardioversion in patients with underlying LV dysfunction as patients may not be able to tolerate it for long. Even when a decision is made for DC cardioversion, while preparing in a hemodynamically stable patient, CSM or IV adenosine may be tried. The procedure can terminate SVT with aberrancy and some VTs — such as outflow tract VT and fascicular VT. IV adenosine if given in antidromic AVRT has a theoretical risk of precipitating AF and accelerated conduction through the accessory pathway resulting in VF. I/V Amiodarone may be a safe option in a wide QRS tachycardia if the patient is hemodynamically stable. Subsequent management depends on the underlying pathology. A VT needs to be evaluated and a lot of patients will require an AICD for secondary prevention of SCD if there is a structural heart disease. In VT with normal left ventricle (LV) EF, further evaluation with a cardiac MRI to see for any scar, fibrosis, infiltrative pathology needs to be done. Sometimes EPS is required to be done to decide further management. RFA using 3D Mapping systems &/ or AICD implantation may be the options of long-term management [19, 20].
Conclusion
Bradyarrhythmia is a common clinical finding. The clinician must determine the relation between bradyarrhythmia and symptoms and differentiate between physiologic and pathologic conditions. In symptomatic and irreversible bradyarrhythmia, pacemaker therapy is highly effective for the relief of symptoms. Tachyarrhythmias like SVT include all forms of arrhythmias that either arise above the bifurcation of the bundle of His or that have mechanisms dependent on the bundle of His. Arrhythmias causing hemodynamic instability (hypotension, heart failure, or coronary ischemia) require urgent electrical cardioversion. However, it is often unclear, especially in the case of atrial fibrillation, whether the supraventricular tachycardia is the cause or the result of the hemodynamic instability. Normal heart VT is generally hemodynamically stable and have a very good success rate with RF Ablation.
Conflicts of interest
Author declares no conflicts of interest.
References
[1] Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122(Suppl 3):S729–S767 [Erratum, Circulation 2011;123(6):e236].
[2] Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med. 2006; 173(8):910–916.
[3] Becker H, Brandenburg U, Peter JH, Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995; 151(1):215–218.
[4] Katritsis DG, Josephson ME. Differential diagnosis of regular, narrow-QRS tachycardias. Heart Rhythm. 2015; 12(7):1667–1676.
[5] Page RL, Joglar JA, Caldwell MA, Calkins H, Al-Khatib SM, et al. 2015 ACC/AHA/ HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016; 67(13):e27–115.
[6] Lee G, Sanders P, Kalman JM. Catheter ablation of atrial arrhythmias: state of the art. Lancet. 2012; 380(9852):1509–1519.
[7] Bun SS, Latcu DG, Marchlinski F, Saoudi N. Atrial flutter: more than just one of a kind. Eur Heart J. 2015; 36(35):2356–2363.
[8] Zhou GB, Hu JQ, Guo XG, Liu X, Yang JD, et al. Very long-term outcome of catheter ablation of post-incisional atrial tachycardia: role of incisional and non-incisional scar. Int J Cardiol. 2016; 205:72–80.
[9] Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012; 60(7):628–636.
[10] Bhatia A, Sra J, Akhtar M. Preexcitation syndromes. Curr Probl Cardiol. 2016; 41(3):99–137.
[11] Mangrum JM, DiMarco JP. The Evaluation and Management of Bradycardia. N Engl J Med. 2000; 342(10):703–709.
[12] Kusumoto FM, Schoenfeld MG, Barrett C, Edgerton JR, Ellenbogen KA, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay. J Am Coll Cardiol. 2019; 140(8):e382–e482.
[13] Michael Glikson, Jens Cosedis Nielsen, Mads Brix Kronborg, Yoav Michowitz, José Maria Tolosana, et al. ESC Scientific Document Group, 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). European Heart Journal. 2021; 42(35):3427–3520.
[14] January CT, Wann LS, Calkins H, Chen LY, Yancy CW, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019; 74(1):104–132.
[15] Katritsis DG, Boriani G, Cosio FG, Jaïs P. Executive summary: European Heart Rhythm Association Consensus Document on the Management of Supraventricular Arrhythmias. Arrhythmia & Electrophysiology Review. 2016; 5(3):210–214.
[16] Page RL, Joglar JA, Caldwell MA, Calkins H, Al-Khatib SM, et al. 2015 ACC/AHA/ HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016; 67(13):e27–115.
[17] Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991; 83(5):1649–1659.
[18] Vereckei A, Duray G, Szénási G, Altemose GT, Miller JM. Application of a new algorithm in the differential diagnosis of wide QRS complex tachycardia. Eur Heart J. 2007; 28(5):589–600.
[19] Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114(10):e385–e484.
[20] Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Page RL, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018; 72(14):e91–220.