Full Text
The term aortic aneurysm (AA) refers to a pathologic segment of aortic dilation that has a propensity to expand and rupture. An increase in diameter of at least 50% greater than expected for the same aortic segment in unaffected individuals of the same age and sex is considered as an aortic aneurysm [1]. Aortic aneurysms are usually described in terms of their size, location, morphology, and cause. Size criteria are focused on cross-sectional diameter as measured on imaging studies. AA are either fusiform or saccular. Thoracic AA are less common compared to abdominal AA due to increased amounts of elastin present in the thoracic aorta compared to the abdominal aorta [2]. Thoracic AAs have an estimated incidence of at least 5 to 10 per 100,000 person-years. Aortic root or ascending aortic aneurysms are most common (≈60%), followed by aneurysms of the descending aorta (≈35%) and aortic arch (<10%) [3]. Thoracoabdominal aortic aneurysm refers to descending thoracic aneurysms that extend distally to involve the abdominal aorta.
Case summary
A 69-year-old male, former smoker (30 pack years), occasional tobacco chewer, no history of alcohol use, no past history of any major medical illness, his general examination was unremarkable with a normal arm span to height and upper segment to lower segment ratios. His family history was negative for any diseases with familial predisposition. He underwent biopsy of a tongue ulcer which revealed squamous cell carcinoma and was planned surgery for the same. During routine preoperative evaluation a chest X ray was done which showed a large dilated aorta (Figure 1). 2D Echo and ECG done were normal.
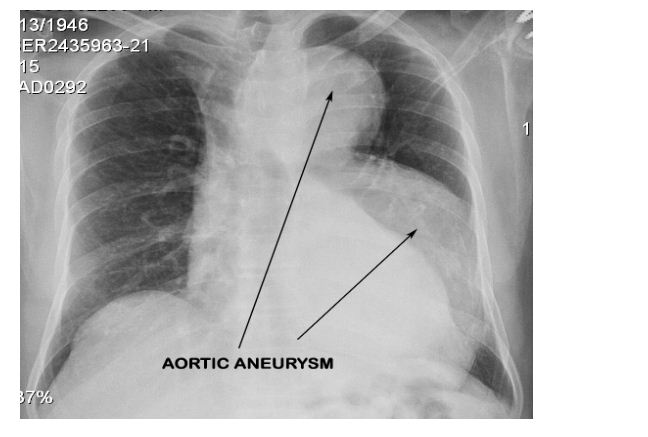
Figure 1: Routine Chest X-Ray that revealed a dilatation of the arch and descending aorta.
CECT aortogram was performed which revealed a large fusiform aortic aneurysm involving the arch and descending thoracic aorta which was impending rupture (Figures 2 & 3).
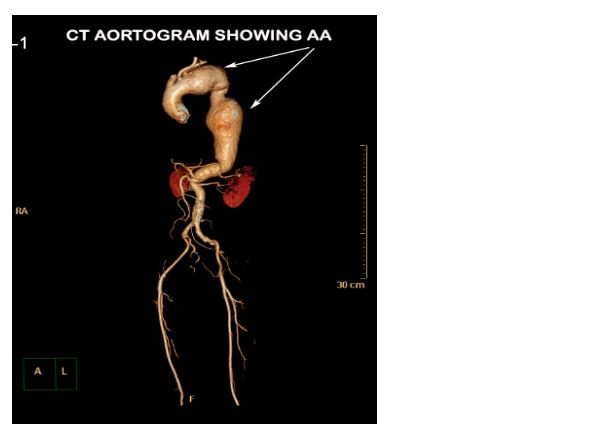
Figure 2: CT aortogram with 3D reconstruction showing the aortic aneurysm (AA).
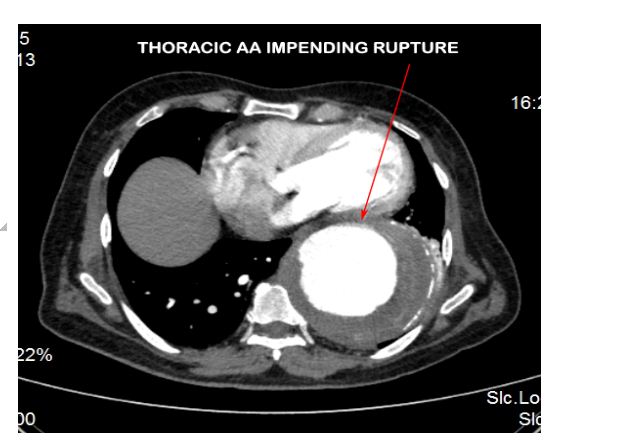
Figure 3: CT aortogram showing the descending thoracic AA impending rupture.
Total arch replacement was initially considered by conventional open surgical approach. However the mortality rate with elective open Thoracic AA repair has been reported to be as high as 19% at 30 days and 31% at 1 year [4]. A hybrid procedure was planned by the heart team that involved an initial debranching of neck vessels from the aortic arch followed by endovascular aneurysm repair (EVAR) of the AA. Initial debranching of neck vessels is done to create an adequate landing zone for the stent grafts to be used for subsequent EVAR. The debranching and diversion of the neck vessels to a zone proximal to the proposed landing zone for the stent graft maintains their ostial patency and ensures adequate circulation. Landing zone is the portion of the aorta where the stent graft anchors so as to prevent its migration. Inadequate landing zones can cause a failure of the stent graft to anchor leading to complications including stent migration. Since the ascending aorta is subject to high pressures from the left ventricle there is a risk of stent migration even with an adequate landing zone. Hence a banding of the ascending aorta was also planned to reduce the pressures and also provide a good anchorage which would reduce chances of migration of the stent graft.
A cerebrospinal fluid (CSF) drain was placed in the L3/ L4 space by the anesthesiologist so as to prevent spinal cord complications as per current practice guidelines. Debranching procedure was carried out by the CT surgery team via a midline sternotomy incision. 14 x 7 mm “Y” grafts were anastomosed to create 4 limbs. The stump of the graft was anastomosed to the ascending aorta using side biting clamp. Ascending aorta was then banded, the innominate artery was transected and the aorta oversewn. The 4 limbs of the conduit were anastomosed to the right subclavian, innominate, left carotid and left subclavian arteries sequentially (Figure 5).
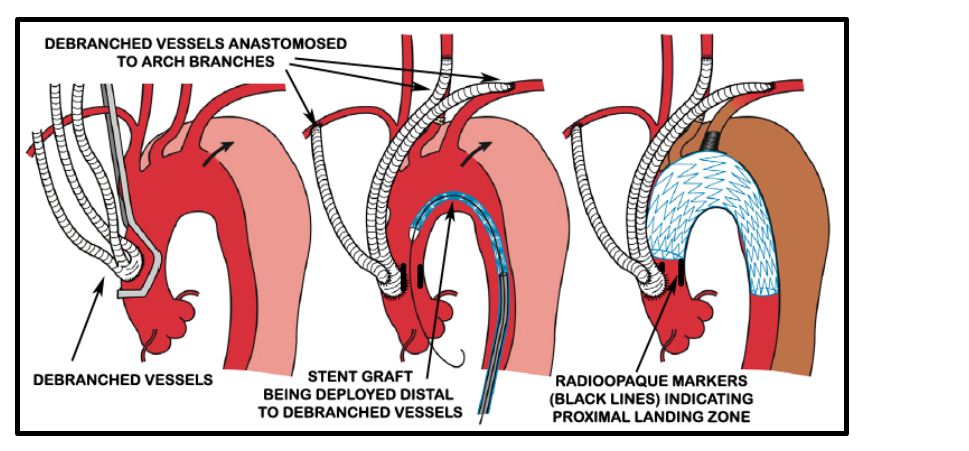
Figure 5: Diagrammatic representation of debranching procedure (Source [5]).
Radioopaque markers were placed in the ascending aorta at the proposed landing zone of the stent graft to indicate the maximal permissible proximal extent of the stent graft to avoid ostial compromise of the debranched vessels (Figure 5).
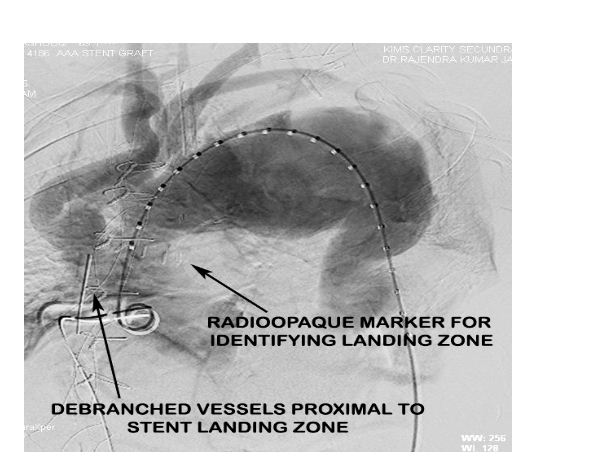
Figure 5: Aortic root angiogram pre-EVAR indicating the debranched vessels and the radiopaque markers placed distal to their site of anastomosis to guide stent graft placement.
Post debranching procedure the patient was monitored and vitals maintained. CSF drain was monitored for pressure and CSF pressure was maintained less than 10 mmHg as per guidelines. The following day after the debranching procedure, the patient was taken up for EVAR procedure by the Cardiology team. Right femoral arteriotomy was done by the vascular surgeon and left femoral artery puncture was done with 6F sheath. After performing an aortic root angiogram via marker pigtail catheter (Figure 6), three stent grafts were deployed in the arch and descending thoracic aorta using the radiopaque markers as a guide for identifying the proximal landing zone for the stent graft (Figure 7).
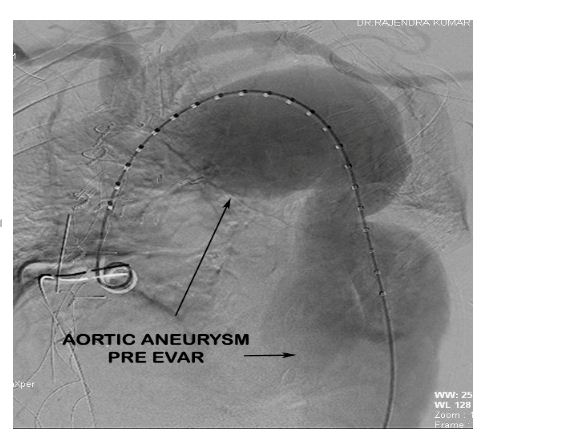
Figure 6: Aortic root angiogram demonstrating the aortic aneurysm pre EVAR.
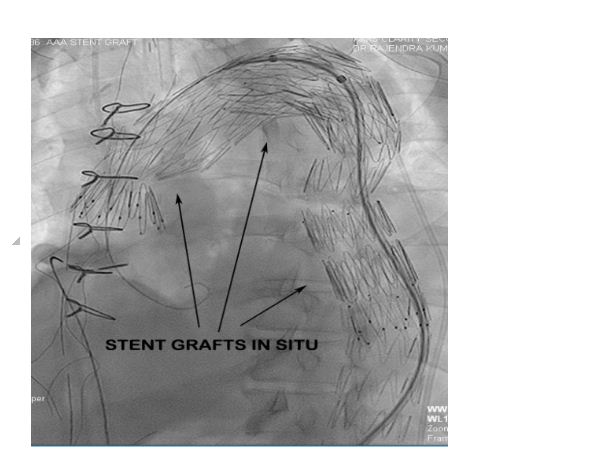
Figure 7: Fluroscopy showing three overlapping stent grafts deployed in arch and descending thoracic aorta.
Procedural result was good with no endovascular leak (Figure 8) post procedure. The patient was started on a single antiplatelet drug (Aspirin 75 mg) post procedure as an antithrombotic agent.
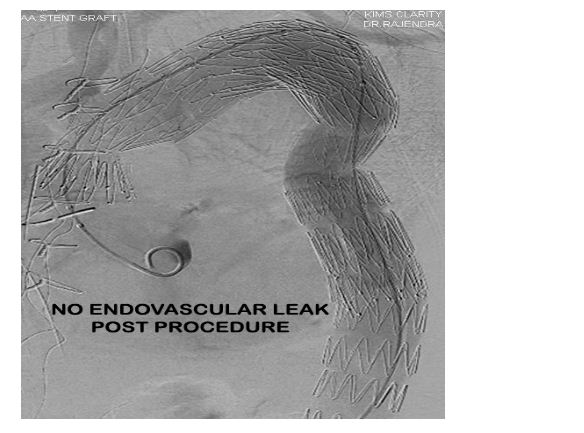
Figure 8: Post procedure aortic angio demonstrating good result and no endovascular leak.
On post procedure day 4 patient developed right sided pleural effusion for which an intercostal drainage tube was placed and 700ml of serosanguinous transudative fluid was drained. Medications were optimised. Drain quantity reduced to minimal over 48 hours and was removed. Patient recovered well, was discharged with no neurological or vascular deficit and is on regular follow up once in three months for six months then once in six months for a year and yearly thereafter.
Discussion
Aortic arch aneurysms especially those involving the mid to distal arch are technically challenging to repair with conventional open techniques. The scenario is further complicated by arch aneurysms frequently extending into either the ascending aorta or descending aorta, or both (so-called “mega aorta syndrome” or “extensive aortic aneurysm”), and may require a two-stage approach for repair. However most of these cases are poor surgical candidates due to advanced age and/or significant comorbidities. Rigberg DA, et al. in a study of 797 patients who underwent elective open thoracic AA repair found a mortality rate of 19% at 30 days and 31% at 1 year [4].
Since the first successful endovascular thoracic aortic aneurysm repair, performed by Dake and colleagues in 1994, endovascular repair has evolved into a valuable treatment for thoracic and aortic aneurysm. This technique is less invasive and equally efficacious, with low morbidity and mortality for high-risk patients, compared to open repair procedures [6]. However, because of anatomic constraints related to required endograft seal zones, a significant number of patients many of whom are not ideal candidates for open surgery, are excluded from standard endovascular repair. In cases of aortic arch or thoracoabdominal aneurysms which often involve the origin of the supra-aortic or visceral-renal branches, it may be necessary to cover those branches using an endovascular stent graft to extend the proximal or distal landing zone. To reduce the risk of neurologic and vascular complications, adjunctive open surgical bypass surgery may be required to provide an adequate landing zone for the stent graft.
Hybrid techniques, including open aortic arch and thoracoabdominal debranching procedures, have been described to allow creation of proximal and/ or distal landing zones for stent graft seal. Current data suggest that these techniques of hybrid debranching and EVAR (H-EVAR) could be performed with lower rates of morbidity and mortality than could conventional, open surgical repair. The staged operation has several advantages, one of which is a less invasive procedure for the patient, shorter operation times and potentially lesser risk of renal failure from contrast load during the operation. Moreover, it is likely that the staged procedure contributes to the preservation of spinal cord perfusion through collateral arterial flow, which may result in lower paraplegia rates [7].
Although EVAR has significantly decreased the overall incidence of neurologic complications with AA repair, the risk of paraplegia with EVAR is reported to be as high as 8%. Various pathophysiological mechanisms have been proposed for spinal cord injury with EVAR including mechanisms related to disruption of radicular artery blood flow to the spinal cord through occlusion of segmental collateral vessels by the endovascular graft or through disruption of other collaterals from the pelvic, lumbar, and hypogastric vessels. Data suggesting that collateral perfusion is of particular importance in the cause of spinal cord injury during EVAR is supportive of the theory that hypoperfusion is the primary etiology of spinal cord injury in these patients. The risk factors for increased incidence of paraplegia following EVAR are patients with previous AA repair, prolonged hypotension, severe atherosclerosis of the thoracic aorta, injury to the external iliac artery, occlusion of the left subclavian artery or hypogastric arteries, and more extensive coverage of the thoracic aorta by the graft, all of which may impede collateral blood flow [8, 9].
A large study by Coselli, et al. demonstrated that lumbar CSF drainage to maintain a CSF pressure less than 10 mmHg leads to an 80% reduction in the incidence of postoperative neurologic deficits (13.0% vs 2.6%, p= 0.03) by improving spinal cord perfusion [10].
Postoperative care for hybrid aortic procedures centers around 2 main concepts: a) hemodynamic stability to ensure adequate organ perfusion, and, b) spinal cord protection. These two concepts are inter-related. Mean arterial pressure is maintained between 80 to 90 mm Hg, with higher goals (90 to 110 mmHg) as there is more extensive coverage of the descending thoracic aorta. Preoperative lumbar drain placement has a definite role in cord protection, especially if the stent graft coverage goes below T6 level, or in patients with previous abdominal aortic aneurysm repair. Intrathecal pressure should be maintained between 10 to 12 mm Hg [11].
Potential advantages of the hybrid approach over conventional repair for arch pathology are: a) These procedures avoid the need for cardiopulmonary bypass and aortic cross-clamping in most patients and have advantages for high-risk patients, including the potential to offer therapy to patients who are not candidates for conventional open repair; b) Endovascular grafts can be deployed from the ascending aorta down to the level of the celiac axis, thus allowing pathology of the arch and descending aorta previously requiring either extensive single stage repair through bilateral thoracosternotomy or two-stage repair to be treated in a single-stage procedure; c) For patients still requiring a two-stage approach, the second-stage endovascular repair may be performed much sooner than a second open procedure, due to the minimal physiologic insult of EVAR and the fact that it is well tolerated even by patients who have recently undergone major open surgery. This represents a significant advance over conventional open second-stage repair and may eliminate the known risk of death from distal aortic complications between stages (interval mortality) [12].
Conclusion
As cardiac teams are treating an increasingly aging population with higher morbidity for conventional surgery, hybrid surgical techniques are gaining more and more relevance. Proper management of AA using hybrid aortic techniques requires an integrated approach by the heart team with experienced open and endovascular skills.
Acknowledgements
Acknowledgements are due to the Departments of Radiology & Imageology, Anaesthesiology and Pulmonology, Krishna Institute of Medical Sciences (KIMS), Secunderabad, for their support in management of this case.
Conflict of Interest
The authors declare no conflict of interest.
References
1. Jain RK, Raju T, Dhall A. Endovascular Management of Aortic Aneurysm and Dissection. Cardiac Catheterisation and Imaging. 2015; 55:876–895.
2. Saratzis A, Bown MJ. The genetic basis for aortic aneurysmal disease. Heart. 2014; 100 (12):916–922.
3. Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J VascSurg. 2012; 56(2):565–571.
4. Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006; 43:217–222.
5. Kuratani T. Best surgical option for arch extension of type B dissection: the endovascular approach. Ann Cardiothorac Surg. 2014; 3(3):292–299.
6. Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007; 133(2):369–377.
7. Matsuno Y, Shimabukuro K, Ishida N, Fukumoto Y, Takemura H. Staged hybrid debranching and thoracic endovascular aneurysm repair for multiple aortic aneurysms after conventional open repair of the descending aorta: a case report. Ann Vasc Dis. 2012; 5(2):225–228.
8. Buth J, Harris PL, Hobo R, van Eps R, Cuypers P, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007; 46(6):1103–1110.
9. Khoynezhad A, Donayre CE, Bui H, Kopchok GE, Walot I, et al. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg. 2007; 83(2):S882–889.
10. Coselli JS, LeMaire SA, Köksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002; 35(4):631–639.
11. Vallabhajosyula P, Szeto WY, Desai N, Komlo C, Bavaria JE. Type II arch hybrid debranching procedure. Ann Cardiothorac Surg. 2013; 2(3):378–386.
12. Hughes GC, Daneshmand MA, Balsara KR, Achneck HA, Sileshi B, et al. “Hybrid” repair of aneurysms of the transverse aortic arch: midterm results. Ann ThoracSurg 2009; 88(6):1882–1887.