Orginal Research
2023
March
Volume : 11
Issue : 1
Histopathological panoroma and clinocopathological correlation of Hansen’s disease in tertiary care hospital
Agrawal S, Sarate D, Agrawal S, Ujade V, Warke SH, Jungare A
Pdf Page Numbers :- 6-9
Shobhana Agrawal1, Dilip Sarate1, Shailesh Agrawal2, Vaishnavi Ujade1,*, Harshal Shankar Warke1 and Ajay Jungare1
1Department of Pathology, Government Medical College, Akola, Maharashtra 444001, India
2Department of T.B Chest, MGM Medical College, Indore, Madhya Pradesh 452001, India
*Corresponding author: Dr. Vaishnavi Ujade, Department of Pathology, Government Medical College, Akola, Maharashtra 444001, India. Email: vaishnaviujade1@gmail.com
Received 10 August 2022; Revised 11 November 2022; Accepted 21 November 2022; Published 2 December 2022
Citation: Agrawal S, Sarate D, Agrawal S, Ujade V, Warke SH, Jungare A. Histopathological panoroma and clinocopathological correlation of Hansen’s disease in tertiary care hospital. J Med Sci Res. 2023; 11(1):6-9. DOI: http://dx.doi.org/10.17727/JMSR.2023/11-2
Copyright: © 2023 Agrawal S et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The present study was undertaken to study the histopathological features of leprosy in skin punch biopsies and to categories them in to various type based on microscopy and bacterial index. The aim of study was to know the role of histopathology in diagnosis of Hansen’s disease and to study the clinicohistopathological correlations of suspected cases of Hansen’s disease.
Materials and methods: A retrospective hospital-based study of clinically diagnosed leprosy cases was conducted over a period of one year (April 2021 to March 2022). Lesional skin biopsies obtained were fixed, processed and stained with Haematoxylin and Eosin (H&E) followed by Fite-Faraco staining. The lesions were classified on microscopy as per Ridley-Jopling classification.
Results: 63 Skin biopsies were obtained from patients with age range of 14-72 years. Highest incidence was in age group of 31 – 50 years. There was male predominance with male to female ratio of 2:1. Clinically borderline Hansen’s (BB) (25.40%) was the most frequent subtype observed followed by borderline lepromatous (BLH) (19.04%) and lepromatous Hansen’s (LL) (19.04%). On histopathology we found tuberculoid Hansen’s (TT) (28.57%) was the most frequently observed subtype followed by borderline tuberculoid Hansen’s (BT) (19.04%). Fite stain revealed lepromatous bacilli in all cases of LL, BL, histoid and indeterminate Hansen’s; whereas bacilli could be demonstrated in six cases of BB, eight cases of BT and two cases of TT.
Conclusion: Early diagnosis of leprosy is clinically difficult as patient present in different clinicopathological form, depending on host immune status. Therefore combined clinical, histopathological and bacteriological features are required for accurate diagnosis and classification.
Keywords: Hansen’s disease; histopathology; bacterial index; clinico-pathological correlation
Full Text
Introduction
Leprosy is chronic granulomatous infection caused by non - cultivable mycobacterium leprae. Mycobacterium leprae is an acid-fast, gram positive bacilli having special affinity for Schwann cell of nerve [1]. It principally affects skin & peripheral nerves but to a varying extent can also involve muscles, eyes, bone, testis and internal organs [2]. Sir Gerhard Armauer Hansen discovered leprosy in the year 1873 [3]. The disease affects individual from early infancy to very old age. The cardinal clinical signs of the disease are hypo pigmented anesthetic patch, thickened peripheral nerves [4]. The incubation period ranges from 2.9 to 5.3 years [5]. Although there has been significant reduction in prevalence of Hansen’s disease, some Southeast Asian countries particularly India & Indonesia indicate continuous transmission.
Leprosy being difficult to diagnose clinically in early stages, histopathology plays a pivotal role in early diagnosis, categorization & treatment to prevent permanent nerve damage & grade 2 deformities. The diagnosis depends upon microscopy & demonstration of acid fast bacilli on tissue biopsy. In case of delayed diagnosis & absent treatment leprosy tends to be progressive & can cause permanent disfigurement. This study was conducted to analyze the histological patterns of Hansen’s disease in skin punch biopsy specimens & to correlate clinicohistopathology & bacteriology. The most widely used being the Ridley-Jopling classification. Ridley & Jopling have proposed the classification of leprosy into five groups as tuberculoid (TT), borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL) and lepromatous (LL) [6].
The aim of study was to know the role of histopathology in diagnosis of Hansen’s disease and to study the clinicohistopathological correlations of suspected cases of Hansen’s disease.
Material and methods
A retrospective hospital based study was conducted in Department of pathology, Government Medical College Akola over a period of one year from April 2021 to March 2022. The study was approved by the institutional ethical committee. Clinically and histopathologically diagnosed patients of leprosy were included. Skin punch biopsies obtained from dermatologist were formalin fixed. Routinely processed and stained with H & E followed by Fite Faraco stain. The lesions were classified as per Ridley – Jopling classification.
Results
A study total 63 cases were included. The age range was from 14 to 72 years. Highest incidence was in age group of 31 – 50 years (Figure 1). Of total 63 cases, 42 (63%) were males and 21 (33%) were females. There was male predominance with male to female ratio of 2:1 (Figure 2).
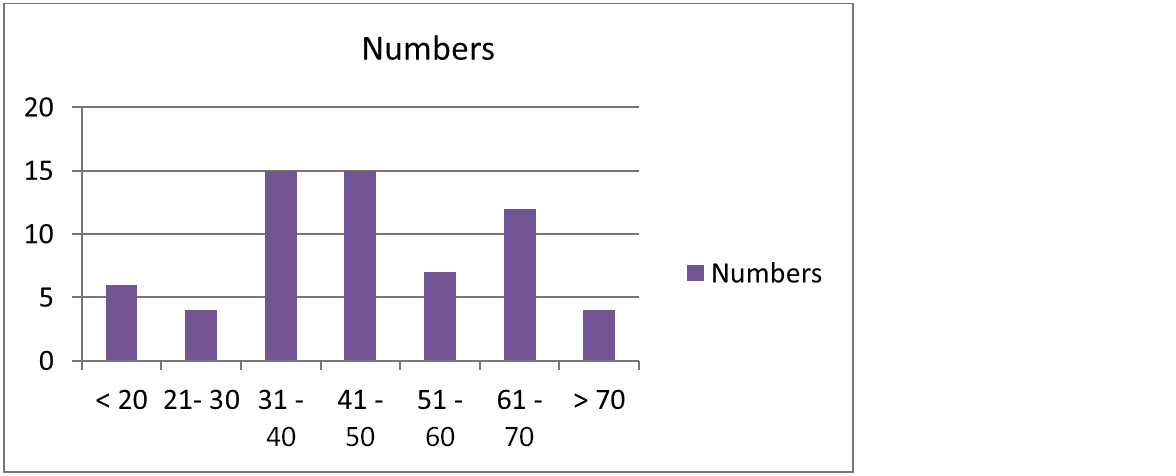
Figure 1: Distribution of Leprosy cases according to age group.
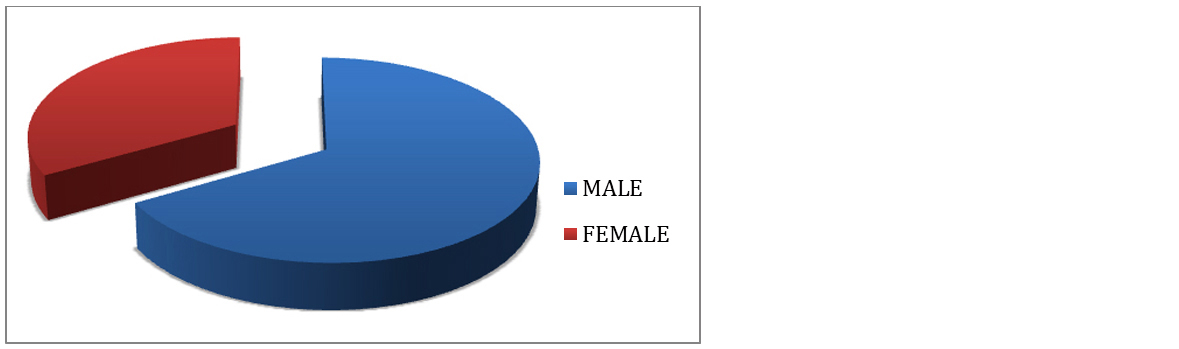
Figure 2: Sex distribution of leprosy cases.
On histopathology, microscopically lesions showing epitheloid cell granuloms with langhans gaint cells and lymphocyted in upper dermis enchroching the dermis were labeled as tuberculoid Hansen (Figure 3) and lesions showing atrophic epidermis, grenz zone and sheets of histiocytes in upper dermis were labeled as lepromatous Hansen (Figures 4 & 5). All cases were correlated with Fite faraco grading. The case with Fite faraco grade 5+ or 6+ were labeled as lepromatous Hansen (Figure 6). Clinically borderline Hansen’s (BB) (25.40%) was the most frequent subtype observed followed by borderline lepromatous (BLH) (19.04%) and lepromatous Hansen’s (LL) (19.04%). On histopathology we found tuberculoid Hansen’s (TT) (28.57%) was the most frequently observed subtype followed by boderlined tuberculoid Hansen’s (BT) (19.04%) (Table 1).
Table 1: Histopathological & clinical correlation of leprosy cases.
|
Clinical diagnosis
|
Histopathology diagnosis
|
|
LL
|
BL
|
BB
|
BTH
|
TT
|
Histoid
|
IND
|
Total
|
|
LL
|
9
|
1
|
-
|
-
|
2
|
-
|
-
|
12
|
|
BL
|
-
|
10
|
2
|
-
|
-
|
-
|
-
|
12
|
|
BB
|
-
|
-
|
6
|
2
|
8
|
-
|
-
|
16
|
|
BTH
|
-
|
-
|
-
|
8
|
3
|
-
|
2
|
13
|
|
TT
|
-
|
-
|
-
|
2
|
5
|
-
|
-
|
7
|
|
Histoid
|
-
|
-
|
-
|
-
|
-
|
3
|
-
|
3
|
|
Total
|
9
|
11
|
8
|
12
|
18
|
3
|
2
|
63
|
TT and BT were most commonly seen in age group of 51 – 70 years where as BL, LL and histoid Hansen’s were seen in younger age group of 31 – 50 years (Table 2). Fite stain revealed lepromatous bacilli in all cases of LL, BL, histoid and indeterminate Hansen’s; whereas bacilli could be demonstrated in six cases of BB, eight cases of BT and two cases of TT. We found 6+ grade in 100% of histoid leprosy, 77% of lepromatous leprosy and 36% of borderline lepromatous leprosy. 5+ grade is seen in 33% of lepromatous leprosy; 4+ grade in 54% of borderline lepromatous leprosy and 25% of borderline leprosy And 3+ grade in 41% in borderline tuberculoid leprosy, 37% of borderline leprosy and 9% of borderline lepromatous leprosy. 1+ grade is found in 25% of borderline tuberculoid leprosy, 11% of TT, 12.5% of BB and 100% of indeterminate Hansen’s. Acid fast bacilli were not demonstrated in 88% of TT, 33% of BTH and 25% of BB (Table 3).
Table 2: Distribution of leprosy cases according to age group.
|
Age group
|
Type of Hansen’s
|
|
LL
|
BL
|
BB
|
BTH
|
TT
|
Histoid
|
IND
|
|
10-20
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
21-30
|
-
|
2
|
-
|
-
|
2
|
-
|
-
|
|
31-40
|
4
|
3
|
2
|
-
|
4
|
2
|
-
|
|
41-50
|
4
|
3
|
-
|
3
|
2
|
1
|
2
|
|
51-60
|
1
|
1
|
-
|
5
|
-
|
-
|
-
|
|
61-70
|
-
|
-
|
2
|
2
|
8
|
-
|
-
|
|
>70
|
-
|
2
|
2
|
-
|
-
|
-
|
-
|
|
Total
|
9
|
11
|
8
|
12
|
18
|
3
|
2
|
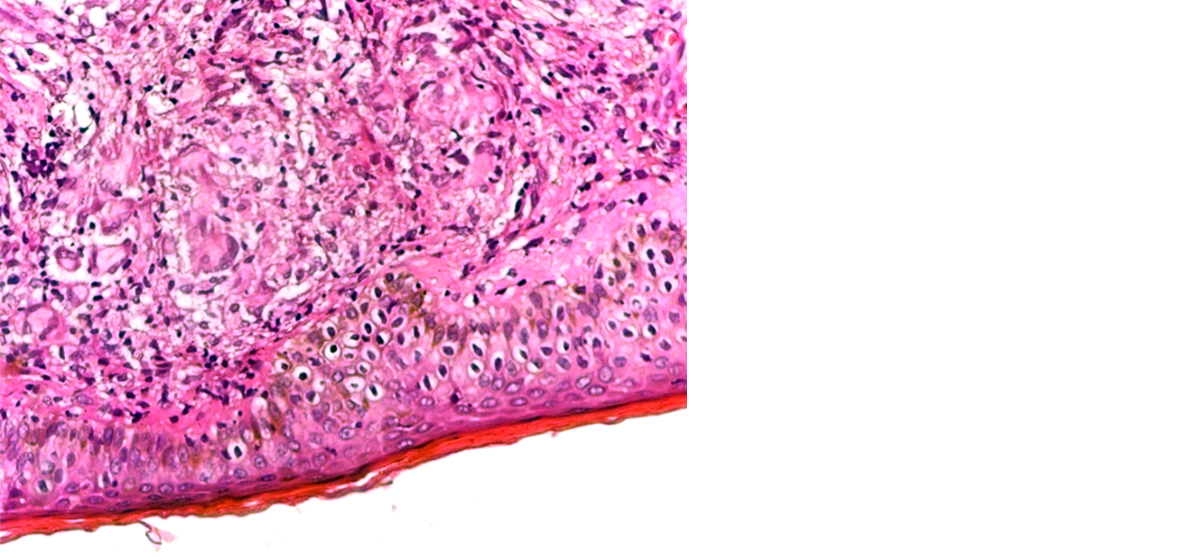
Figure 3: Tuberculoid leprosy with epitheloid granuloma in dermis enchroching epidermis. Langhans giant cells were also seen at places (40X).
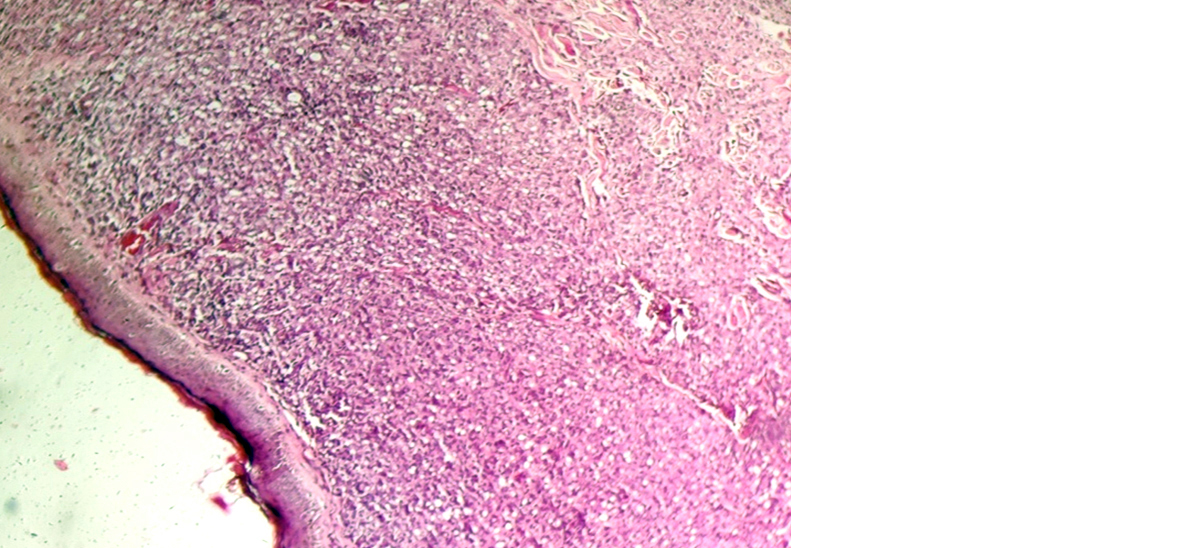
Figure 4: Lepromatous leprosy showing atrophic epidermis with grenz zone and foamy histiocytes in dermis (10X).
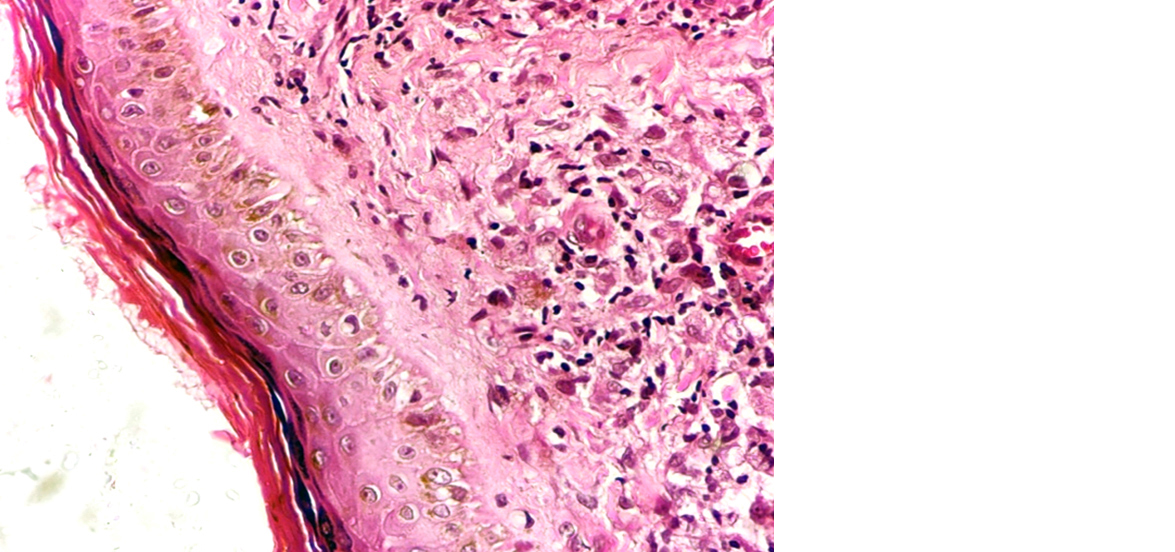
Figure 5: Lepromatous leprosy showing atrophic epidermis with grenz zone and foamy histiocytes in dermis (40x).
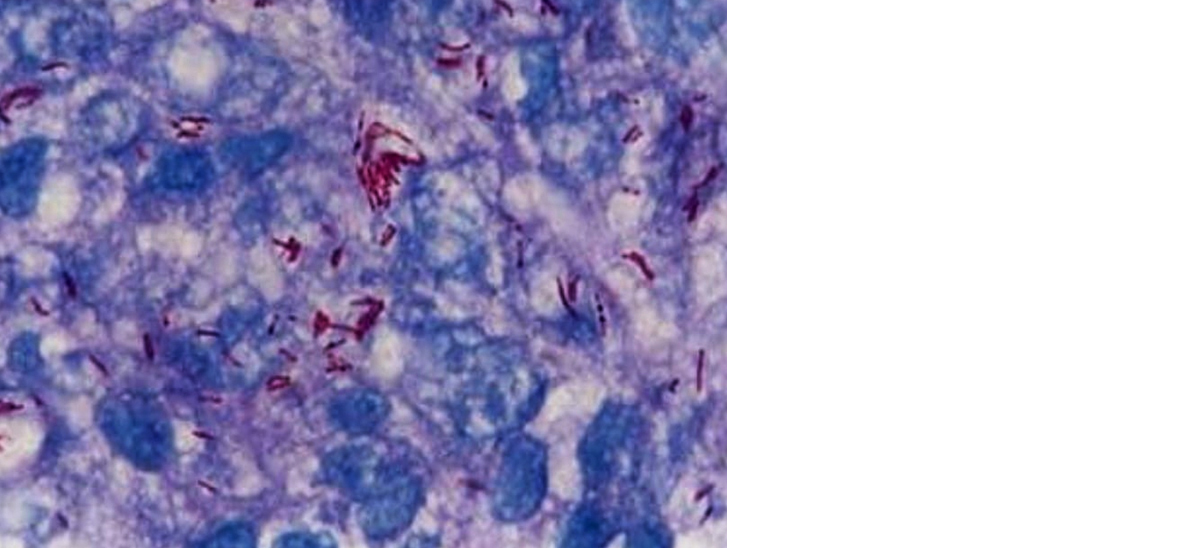
Figure 6: Fite Faraco stain showing lepra bacilli in globi in case of lepromatous leprosy (100X).
Discussion
Hansen’s disease is slowly progressive chronic infectious disease. The mode of transmission is probably the inhalation of bacilli. After inhalation bacilli passes from blood to peripheral & cutaneous nerves where infection & host reaction occurs. The clinic-pathologic manifestations are the result of immunopathology & accumulation of infective cells. Tuberculoid leprosy indicates high cellular immune response & few bacilli in tissue, at the opposite pole lepromatous leprosy indicate absent of cellular immune response to mycobacterium leprae Ag & abundant bacilli in tissue.
With the introduction of multidrug therapy (MDT), elimination of leprosy, which is defined as <1 case per 10,000 population, was achieved at global level by the year 2000. However, the new case detection rate and disease burden associated with leprosy are still significant in endemic countries [7]. Delayed diagnosis and slow recognition of reactional episodes in turn leads to more severe nerve damage and scarring [8, 9]. In cases where skin lesions are subtle or inconclusive, laboratory investigations are warranted for confirmation of the disease [10]. The histological examination particularly is more significant in group of leprosy patients (BT+BB+BL) where the immune status is continuously shifting [11]. The histological diagnosis gives better information about the subtype and any shift in the spectrum of the disease. Thus, it is important to study the skin biopsy from morphological lesion and serial biopsies from same or paired lesions to achieve better clinicopathological correlation [12, 13].
In present study, males (67%) were more affected than females (33%) as found in studies done by Sinha et al [14] in which affected males were 71% and female were 29% and Dhakwa et al [3] in which affected males were 70% and females were 30%.
In the studies done by Sinha et al [14] and Srismitha [15] most common age group affected was 31-50 years which was similar to our study whereas in the studies done by Atram et al [16] found majority of cases in age group was 21-40 years.
Present study shows the histopathologically most common subtypes of leprosy were TT and BT which are similar to the study of Srismitha et al [15], Dhakwa et al [3] and Sushilkumar et al [17]. In study done by Nila Theresa Davis et al [18] BT was the most common subtype found followed by BL.
Like our study, few studies done by Shushilkumar et al [17] and Davis et al [18] 6+ grade is found in all cases of histoid Hansen’s and maximum cases of LL whereas acid fast bacilli were not demonstrated in maximum cases of TT.
Limitation of study
This data was collected in tertiary care centre in limited patients. Multi-centre with more patients gives precise results.
Conclusion
Early diagnosis of leprosy is clinically difficult as patient present in different clinico-pathological form, depending on host immune status. Therefore combined clinical, histo-pathological and bacteriological features are required for accurate diagnosis and classification. Accurate diagnosis forms the backbone for appropriate treatment and preventing deformities and drug resistance.
Acknowledgement
All our patients and technical staff.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Kumar U, Varma K, Baraithiya K. Clinico-epidemiological and histopathological study of newly diagnosed leprosy patients at tertiary care center. IP Indian J Clin Exp Dermatol. 2020; 6:374–381.
[2] Kumar B, Rai R, Kaur I. Systemic involvement in leprosy and its significance. Indian J Lepr. 2000; 72:123–142.
[3] Dhakhwa R, Majagaiyan S, Pradhan S. Histopathological study of Hansen’s disease. BJHS. 2021; 6:1606–1610.
[4] Banu OAM, Uma R. Clinicopathological correlation study of different spectrum of Hansen’s disease. MedPulse – Int Med J. 2016; 3:531–535.
[5] Prasad KV, Ali PM. Incubation period of leprosy. Indian J Med Res. 1967; 55:29–42.
[6] Mathur MC, Ghimire RBK, Shrestha P, Kedia SK. Clinicohistopathological Correlation in Leprosy. Kathmandu Univ Med J. 2011; 9:248–251.
[7] Global leprosy update, 2013; reducing disease burden. Wkly Epidemiol Rec. 2014; 89:389–400.
[8] Rodrigues LC, Lockwood DN. Leprosy now: Epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011; 11:464–470.
[9] Yadav N, Kar S, Madke B, Dashatwar D, Singh N, et al. Leprosy elimination: A myth busted. J Neurosci Rural Pract. 2014; 5:S28–S32.
[10] Chan MMF, Smoller BR. Overview of the Histopathology and Other Laboratory Investigations in Leprosy. Curr Trop Med Rep. 2016; 3:131–137.
[11] Bindal T, Kalra IK, Garg M, Mahindra A, Nangia R. Clinical and histological profile of leprosy patients at rural based tertiary care centre in post elimination era. Ann Pathol Lab Med. 2018; 5:A289–A295.
[12] Bhatia AS, Katoch K, Narayanan RB, Ramu G, Mukherjee A, et al. Clinical and histopathological correlation in the classification of leprosy. Int J Lepr Mycobact Dis Off Organ Int Lepr Assoc. 1993; 61:433–438.
[13] Sandeep M, Murugesh S. A study of clinico-pathological concordance in leprosy patients in the post-elimination era. Int J Sci Res. 2016; 5: 1304–1306.
[14] Sinha R, Kumari M, Bhadani PP. Histopathological panorama of leprosy in a tertiary care hospital of Bihar. J Clin of Diagn Res.2019; 13:EC12–EC15.
[15] Srismitha S, Karthik S, Shobana B, Manjani S. Clinico-histopathological correlation in Hansen’s disease. Saudi J Pathol Microbiol. 2019; 6:258–263.
[16] Atram MA, Ghongade PV, Gangane N. A clinicohistopathological correlation of Hansen’s disease in a rural tertiary care hospital of central India. J Global Infect Dis. 2020; 12:191–196.
[17] Sushilkumar KS, Rekha RI, Darshana UP, Supriya B, Seema B. Histopathology and Clinico-histopathological correlation in Hansen’s disease. J Res Med Dent Sci. 2014; 2:37.
[18] Davis NT, Letha V, Sankar S. Histopathology and bacteriology in Hansen’s disease. J Evol Med Dent Sci. 2017; 6:6492–6496.