Full Text
Hypertrophic cardiomyopathy (HCM) is a complex muscular disorder of the heart characterized by left and/or right ventricular asymmetric hypertrophy involving the interventricular septum. It is inherited as an autosomal dominant disease in at least 50% of the cases, though sporadic cases due to de novo mutations are also observed. The condition is characterized by a heterogeneous disease expression with the prevalence reported to be 1 in 500 of the population [1-2].
Based on the pattern and extent of hypertrophy and obstruction, HCM is further classified into five types: (Figure 1) Asymmetric septal hypertrophy (ASH), 2) Obstructive hypertrophic cardiomyopathy (HOCM) 3) Non-obstructive HCM 4) Apical HCM 5) Mid- cavity concentric HCM.
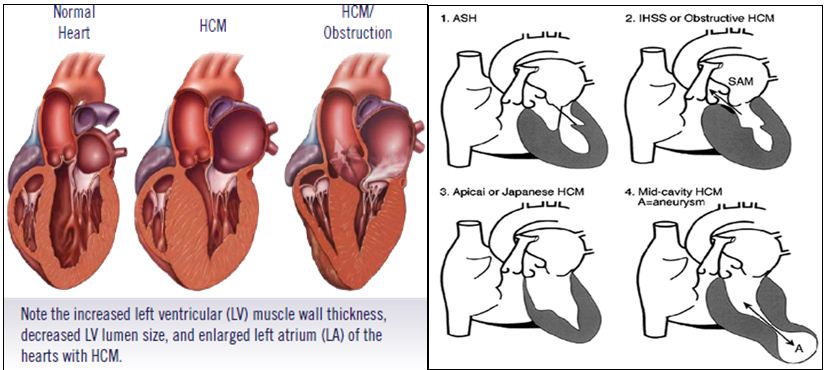
Figure 1: Subtypes of hypertrophic cardiomyopathy.
Pathological features of HCM are: cardiac myocyte hypertrophy and disarray, interstitial fibrosis and thickening of intra-mural coronary arteries. Myocyte disarray is considered to be the pathological hallmark of HCM (Figure 2).
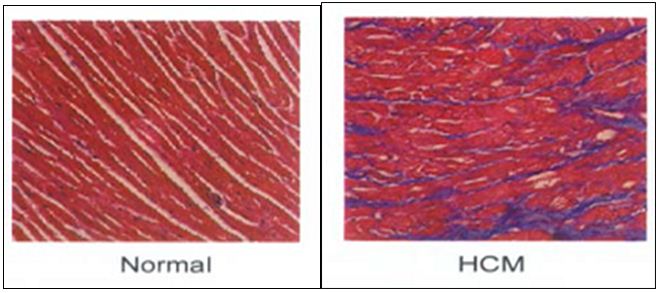
Figure 2: Histopathology of the heart showing myocardial disarray.
It exhibits great clinical variability from being asymptomatic to severe heart failure with the manifestation of the disease in the 4th/ 5th decade of life. The phenotypic variability can be attributed to compound heterozygosity, modifier genes, epigenetic factors (DNA methylation and imprinting), epistasis (interaction between genes), post-transcriptional and post-translational modifications of gene products, presence of coexisting diseases, and environmental influences [3].
HCM has been widely studied, and a range of population based approaches have assessed the prevalence and incidence of HCM. The estimates varied almost by ten-fold which could be attributed to ethnicity, differences in populations, study methods and settings. The studies showed a consistent male preponderance compared to females at all ages below 70 years, by a factor of two to three [4]. The epidemiology and natural history of HCM is incompletely understood, particularly with respect to the risk of sudden death. Epidemiological studies based on tertiary referral centers report higher deaths than community based studies. The most relevant, recent UK based study by Wald et al. (2004) provides some reassurance on fatality in undiagnosed HCM, at about 6/10,000 per year [5].
Apart from the mutations in sarcomeric and cytoskeletal genes implicated in the manifestation of the disease, gender specific differences also contribute to the variable expressivity of the disease. Therefore, present study gives the epidemiological and clinical analyses of HCM patients emphasizing on gender specific differences.
Methodology
HCM diagnostic criteria
The HCM patients were diagnosed based on the physical examination, ECG and ECHO findings. Table 1 gives the major and minor criteria in diagnosing HCM. The individual need to fulfil any of the 3 criteria’s to be diagnosed with HCM, i.e. i) 2 major criteria, ii) 1 major + 1 minor criteria, and iii) 1 electrocardiography + 1 echocardiography criteria.
Table 1: Diagnostic criteria for hypertrophic cardiomyopathy.
|
Echocardiography
|
Major criteria
|
Minor criteria
|
|
Left ventricular wall thickness in the anterior septum
|
≥13mm
|
≥12mm
|
|
Posterior septum or free wall
|
≥15mm
|
≥14mm
|
|
|
Severe SAM (systolic anterior motion of mitral valve)
|
Moderate SAM
|
|
Electrocardiography
|
LVH + repolarization changes
|
Complete bundle branch block or minor interventricular conduction defects
|
|
|
T wave inversion in leads I and a VL
|
Minor repolarization changes in LV Leads
|
|
|
Abnormal Q wave in at least 2 leads
|
Deep S wave in V2
|
|
Clinical features
|
Unexplained dyspnea, chest pain or syncope
|
Sample size and source
100 HCM cases (76 males and 24 females) were included in the study referred from the cardiology units of CARE hospitals (Nampally, Banjara Hills, and Secunderabad) while the control group collected from Osmania General Hospital, Hyderabad, constituted 60 males and 40 females. The epidemiological variables like age, sex, age at onset, family history, and parental consanguinity were noted. The clinical data from the patients and their relatives were collected after taking the informed written consent, which included the collection of latest report of electrocardiogram (ECG) and echocardiogram (ECHO) along with the observed symptoms and information of the family history. The study was approved by the institutional ethical committee of CARE Hospitals. Informed written consent was also obtained from participating patients and their family members. Figures 3a and 3b give electrocardiogram of a normal individual and HCM patient.
Results and discussion
Table 2 gives the epidemiological characteristics and familial/non-familial status of the HCM patients. The study revealed a higher male preponderance with a sex ratio of 3.1:1. Familial HCM cases were observed to be higher in females, attributing to severe clinical symptoms and poor prognosis. The mean age at onset was observed to be 38.8± 13.6 years in males when compared to 38.4±18.1 years in females with no significant difference.
Table 2: Epidemiological characteristics in HCM.
|
Parameter
|
Males N (%)
|
Females N (%)
|
|
Controls
|
60 ( 60)
|
40 ( 40)
|
|
HCM
|
76 (76)
|
24 (24)
|
|
Sex ratio
|
3.1:1
|
|
|
Mean age at diagnosis (mean±SD)
|
45.5±13.8
|
41.2±20.2
|
|
Mean age at onset (mean±SD)
|
38.8±13.6
|
38.4±18.1
|
|
Familial
|
44 (57.8)
|
16 66.6 (1:1.1)
|
|
Non-familial
|
32 (42.1)
|
8 33.3 (1:1.2)
|
|
Consanguinity
|
4 (5.2)
|
2 (8.3)
|
|
Diabetes
|
12 (15.7)
|
2 (8.3)
|
|
Hypertension
|
25 (32.8)
|
5 (20.8)
|
|
Smoking/alcohol
Ex-smoker
Smoker
Ex-alcoholic
Occasional drinker
|
14 (18.4)
5 (6.5)
9 (11.8)
13 17.1
|
Nil
|
Table 3 gives distribution of age at onset with respect to gender, wherein the cohort was subdivided into 3 age groups: early (0-20 years), middle (21-40 years) and late (41-60 years) onset. Higher number of male cases was observed in the middle age group of 21-40 years. Interestingly, female preponderance was observed in early (0-20 years) and late (41-60 years) onset groups.
Male preponderance could be attributed to various factors like developmental, anatomical, hormonal and environmental triggers. These differences could confer greater vulnerability to hypertrophic stimuli and left ventricular stress, diastolic and systolic dysfunctions. Additionally, addiction of smoking and alcohol can also add to the severity and progression of HCM. Delayed onset of symptoms in females make nearly one-third of women remain asymptomatic till the fourth decade, with symptoms developing only after the fifth decade of life. They show blunted cardiac responses because of inactivation/over expression of certain genes. The female hormone; estrogen plays an important role in delaying the onset of the symptoms by protective cardiovascular mechanisms wherein the activation of estrogen receptor might modulate the hypertrophic signalling [6]. Thus age at onset is a considerable parameter that further supports the heterogeneity of HCM.
Table 3: Gender specific distribution of age at onset.
|
Age at onset (years)
|
Males (n=76)
|
Females (n=24)
|
M:F Total
|
|
0-20
|
5 (6.5%)
|
4 (16.6%)
|
1.2:1 9%
|
|
21-40
|
30 (39.4%)
|
5 (20.8%)
|
6:1 35%
|
|
41-60
|
41 (55.2%)
|
15 (66.6%)
|
2.7:1 56%
|
Table 4 gives the frequency distribution and age at onset based on HCM subtypes in males and females, wherein the non-obstructive hypertrophy was observed to be predominant in males while the obstructive HCM cases were observed to be predominant in females. This was in concordance with the study carried out by Woo et al. (2005), wherein HOCM was reported to be more common in females than males [7]. Further the subtype HOCM exhibits strong familial tendency and high penetrance compared to other subtypes. Predominance of obstructive type of HCM in females could be attributed to the fact that plaque along the walls of coronary arteries accumulates more diffusely in women blocking the heart vessels more evenly which could be related to small size and higher contractility of the left ventricle, whereas in males large clumps are formed [8, 9].
Olivotto et al. (2005) reported a higher risk of progression to advanced heart failure or death associated with outflow obstruction in female patients since they were observed to be under-represented, older, and more symptomatic with poor prognosis [6]. Other possibilities include an enhanced susceptibility of female patients to HCM due to the consequences of atrial fibrillation, embolic stroke, and left ventricular remodelling. These gender specific differences suggest that social, endocrine, or genetic factors may affect the diagnosis and clinical course of HCM.
The subtypes of HCM clearly reveal the heterogeneous condition of the disease with gender specific distribution. However, the difference cannot be attributed totally to the known demographic and pathophysiologic variables, but factors specific to the female gender may also play an important and crucial role in risk stratification. Women may be more prone to disease progression due to a variety of physiologic factors which are age related, showing limited response to treatment and exhibiting increased morbidity. Differential presentation of symptoms reflects differences in the underlying etiology and characteristics of the disease [10]. Although the mechanisms underlying these gender differences in the incidence and progression of disease are largely unknown, the role of sex hormones in modulating the activity of several regulatory systems, including the renin-angiotensin system (RAS), has been suggested along with variable genetic predisposition [11].
Table 4: Frequency distribution and age at onset based on HCM subtypes.
|
Type of HCM
|
Males (n = 76)
|
Mean age at onset (years)
|
Females ( n = 24)
|
Mean age at onset (years)
|
|
HCM/ non-obstructive HCM
|
40 (52.6%)
|
40.0±11.0
|
9(37.5%)
|
37±15.9
|
|
Obstructive HCM
|
17 (22.3%)
|
35.8±19.3
|
9 (37.5%)
|
40.1±18.8
|
|
Asymmetric septal hypertrophy
|
12 (15.7%)
|
37±14.5
|
5 (20.8%)
|
27.3±9.10
|
|
Apical hypertrophy
|
5 (6.5%)
|
42.4±8.45
|
1(4.1%)
|
52±0
|
|
Mid cavity concentric hypertrophy
|
2 (2.6%)
|
29.5±8.5
|
---
|
--
|
Females with asymmetric septal type of hypertrophy were found to have an early age at onset while in males early onset was observed in Mid-cavity concentric hypertrophy. Delayed onset was observed in Apical HCM irrespective of the gender. The different subtypes of HCM tend to show variable disease onset almost differing by a decade which could be attributed to the male and female anatomical and hormonal variations and the underlying genetic factors. The modifier genes might also have a gender specific effect resulting in heterogeneity of the disease. Asymmetric septal hypertrophy primarily involves the interventricular septum. Histologically, HCM is characterized by disordered cardiac architecture and myocardial cells that are largely present in the interventricular septum of patients with asymmetric septal hypertrophy and left ventricular obstruction. When this is observed in female patients, it leads to severe expressivity of the symptoms resulting in early disease onset.
Apical HCM is characterized by hypertrophy in only the apex of the left ventricle and is predominantly observed in males [12]. It is mostly asymptomatic in the initial stages but manifests later in life. In the present study, a single case of apical HCM was observed in a female with a delayed onset of 52 years, which reinforces the fact that apical HCM is a relatively benign and late onset disease.
In case of mid cavity HCM, the hypertrophy is mainly at the level of the papillary muscles, and a narrowed passage connects the proximal left ventricle with an apical aneurysm leading to obstructions both at the left ventricular outflow tract and in the mid-cavity which may result in an early disease onset, on the other hand non-obstructive HCM do not lead to obstruction of the left ventricular outflow tract resulting in delayed manifestation of the disease. The manifestation of the disease depends on severity of the left ventricular obstruction that would again vary with respect to gender due to varied anatomical attributes, genetic variations modifier genes and intrinsic environmental effects.
The frequency distribution of symptoms in males and females is given in Table 5 wherein the most common presenting symptoms irrespective of the gender were dyspnoea. Symptoms of palpitations were observed to be more common in females (75%) compared to males (56%). This could be explained based on the fact that females have stronger clinical manifestations and are more symptomatic, due to high number of obstructive HCM cases reported, severe mitral regurgitation, diastolic dysfunction and poor prognosis. It could also be noted that the time difference between the age at onset and age at diagnosis was 6 years for males whereas it was only 3 years for females. This difference could be one of the reasons for stronger clinical manifestations in females which was further supported by a study of Olivotto et al. (2005), who reported that female patients were less often diagnosed by routine medical examination and were more symptomatic particularly with dyspnoea and palpitations due to lack of attention to early clinical signs [6]. Besides these factors, delayed HCM diagnosis in women may be secondary to genetic and endocrine factors directly influencing the phenotypic outcome [13-15].
Table 5: Symptomatology in HCM cases.
|
Symptoms
|
Males
(n=76)
|
Females
(n=24)
|
|
Dyspnea
|
57 (75%)
|
18 (75%)
|
|
Palpitation
|
43 (56%)
|
18 (75%)
|
|
Chest pain
|
23 (30%)
|
11 (46%)
|
|
Syncope
|
17 (22%)
|
7 (29%)
|
|
Pre-syncope
|
20 (26%)
|
9 (37%)
|
Table 6 gives the mean of echocardiographic parameters with respect to gender. HCM is characterized by left ventricular hypertrophy (LVH) that is disproportionate to the haemodynamic load. It results in abnormal left ventricular wall stiffness causing impaired diastolic filling [16]. The interventricular septum is a muscular wall that separates the left and right ventricles of the heart. An interventricular septal thickness (IVS) of >1.5cm is considered to be hypertrophic.
In the present study, a significant difference was observed in the Left ventricular outflow tract thickness (LVOT) with a mean of 58.5±29.5mmHg in males and 46.1±24.7mmHg in females. The mean of IVS was also observed to be higher in males 1.93±0.51cms compared to females 1.79±0.46cms. This could be due to slight anatomical variations existing between the two genders. The mean of left ventricular posterior wall (LVPW) and Left ventricular ejection fraction (LVEF) did not show any difference in both males and females respectively.
Table 6: Comparisons of echo characteristics in HCM.
|
Characteristics
|
Mean ± S.D
|
|
Males
|
Females
|
|
Left ventricular posterior wall thickness (LVPW) (cm)
|
1.18±0.24
|
1.11±0.2
|
|
Interventricular septum (IVS) (cm)
|
1.93±0.51
|
1.79±0.46
|
|
Left ventricular outflow tract gradient (LVOT) (mmHg)
|
58.5±29.5
|
46.1±24.7
|
|
Left ventricular ejection fraction (LVEF) (%)
|
68.06±10.09
|
68.3± 7.7
|
Table 7 describes the intra-group comparisons of ECHO characteristics with respect to the subtypes of HCM. The mean LVPW (1.16±0.23cm) and IVS (1.93±0.48cm) were observed to be significantly increased in non-obstructive HCM. In this subtype, the left ventricle is primarily involved which might result in increased contractile wall thickness. Significant mean IVS (1.91±0.48cm) was revealed in asymmetrical septal hypertrophy, as it is primarily characterized by the myocardial disarray of the interventricular septum. In case of mid cavity concentric hypertrophy, the mean LVOT (90±0mmHg) and LVEF (75.5±4.5%) were found to be significant, because the hypertrophy in this type, mainly involves the papillary muscles with a narrow passage connecting the proximal left ventricle leading to obstructions both at the left ventricular outflow tract and in the mid cavity.
Table 7: Comparisons of echo characteristics with respect to subtypes of HCM.
|
Type of HCM
|
LVPW(cm)
|
IVS(cm)
|
LVOT(mmHg)
|
LVEF(%)
|
|
HCM/ non-obstructive
|
1.16±0.23
|
1.93±0.48
|
48.8±23.9
|
67.7±9.3
|
|
Obstructive HCM
|
1.14±0.15
|
1.8±0.47
|
60.5±26.6
|
69.8±9.6
|
|
ASH
|
1.08±0.24
|
1.91±0.48
|
30±21.4
|
66.3±6.5
|
|
Apical HCM
|
0.87±0.16
|
1.6±0.3
|
63±13
|
69.8±1.35
|
|
MOC
|
1.1±0
|
1.75±0.65
|
90±0
|
75.5±4.5
|
To understand the genetic predisposition of each subtype of HCM, the sporadic and familial cases were divided based on the HCM subtypes, as given in Table 8.
Table 8: Familial status based on subtypes of HCM.
|
Type
|
Non-familial (%)
|
Familial (%)
|
F/ho sudden death (%)
|
|
HCM/ non-obstructive (51)
|
22
|
22
|
7
|
|
HOCM (26)
|
7
|
14
|
5
|
|
ASH (15)
|
4
|
10
|
1
|
|
Apical HCM (6)
|
4
|
1
|
1
|
|
MCO (2)
|
--
|
2
|
--
|
|
Pooled (100)
|
37
|
49
|
14
|
Asymmetrical septal hypertrophy and mid-cavity concentric hypertrophy were observed to be rare but still have a strong genetic predisposition while Obstructive and non-obstructive HCM have equal probability of familial and sporadic occurrence. The present study examines the epidemiological and clinical analyses based on gender, age at onset and type of HCM which contributes extensively to the heterogeneity of the disease, making genetic diagnosis and mutational screening more challenging.
Conclusion
The epidemiological and clinical analyses of the present study revealed 37% of sporadic cases and 63% of familial HCM cases, with a sex ratio of 3.1:1, indicating a male preponderance. The mean age at onset of 38 years was observed irrespective of the gender. Interestingly, males were predominant in the age group of 21-40 years exhibiting non-obstructive type of HCM while female preponderance was observed in the age group of 0-20 years and 40-60 years with high incidence of obstructive HCM cases associated with severe clinical manifestations and poor prognosis, based on the significant echocardiographic parameters. This was further substantiated with an early age at onset in females with asymmetric septal type of hypertrophy and in males with mid-cavity concentric hypertrophy enlightening on a strong genetic predisposition. Apical and mid-cavity concentric HCM were reported to be rare in the present study, hence it is observed that gender, age at onset and type of HCM could contribute as epidemiological variables in the disease onset and could account for the heterogeneity with emphasis being laid on the genetic basis and established mode of inheritance.
Acknowledgements
The contribution of all the authors is acknowledged.
Funding
The study has been funded by Department of Science and Technology (DST), New Delhi, India. We thank Indian Council of Medical Research (ICMR) for providing Senior Research Fellowship (2010-2013) to Advithi Rangaraju.
Conflict of interest
The authors declare no conflict of interest.
References
1. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults.echocardiographic analysis of 4111 subjects in the cardia study. Circulation 1995; 92:785–789.
2. Mc Kenna W, Elijah R. Hypertrophic cardiomyopathy: Management risk, stratification and prevention of sudden death behr. Heart 2002; 7:169–176.
3. Marian AJ. On predictors of sudden cardiac death in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 41:994–996.
4. Miura K, Nakagawa H, Morikawa Y, Sasayama S, Matsumori A, et al. Epidemiology of idiopathic cardiomyopathy in Japan: results from a nationwide survey. Heart 2002; 87(2):126–130.
5. Wald DS, Law M, Morris JK. Mortality from hypertrophic cardiomyopathy in England andWales: clinical and screening implications. Int J Cardiol. 2004; 97(3):479–484.
6. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005; 46:480–487.
7. Woo A, William W, Richard C, Duglas W, Evelyn R, et al. Clinical and echocardiographic deteminants of long term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 2005; 111:2033–2041.
8. Dimitrow, Janusz G, Bogumiła B, Dariusz D, Jacek L et al. The Importance of ventricular septal morphology in the effectiveness of dual chamber pacing in hypertrophic obstructive cardiomyopathy. Pacing and Clinical Electrophysiology 2000; 23(9):1324–1329.
9. Kubo T, Kitaoka H, Okawa M, Hirota T, Hayato K, et al. Gender-specific differences in the clinical features of hypertrophic cardiomyopathy in a community-based Japanese population: Results from Kochi RYOMA study. Journal of Cardiology 2010; 56:314–319.
10. Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, et al. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease.Circulation 2003;108:2342–2348.
11. Maric C. Sex differences in cardiovascular disease and hypertension involvement of the renin-angiotensin system. Hypertension 2005; 46:475–476.
12. Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 39: 638–645.
13. Mosca L, Jones WK, King KB, Ouyang P, Redberg RF, et al. Awareness, perception, and knowledge of heart disease risk and prevention among women in the United States. American Heart Association Women’s Heart Disease and Stroke Campaign Task Force. Arch Fam Med. 2000; 9:506–515.
14. Mosca L, Ferris A, Fabunmi R, Robertson RM, American Heart Association. Tracking women’s awareness of heart disease: an American Heart Association national study. Circulation 2004; 109:573–579.
15. Collins P, Stevenson JC, Mosca L. Spotlight on gender. Cardiovasc Res. 2002; 53:535–537.
16. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet 2004; 363:1881–1891.